Identification of glyA (encoding serine hydroxymethyltransferase) and its use together with the exporter ThrE to increase L-threonine accumulation by Corynebacterium glutamicum
- PMID: 12089010
- PMCID: PMC126772
- DOI: 10.1128/AEM.68.7.3321-3327.2002
Identification of glyA (encoding serine hydroxymethyltransferase) and its use together with the exporter ThrE to increase L-threonine accumulation by Corynebacterium glutamicum
Abstract
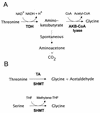
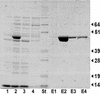
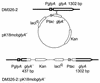
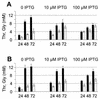
L-threonine can be made by the amino acid-producing bacterium Corynebacterium glutamicum. However, in the course of this process, some of the L-threonine is degraded to glycine. We detected an aldole cleavage activity of L-threonine in crude extracts with an activity of 2.2 nmol min(-1) (mg of protein)(-1). In order to discover the molecular reason for this activity, we cloned glyA, encoding serine hydroxymethyltransferase (SHMT). By using affinity-tagged glyA, SHMT was isolated and its substrate specificity was determined. The aldole cleavage activity of purified SHMT with L-threonine as the substrate was 1.3 micromol min(-1) (mg of protein)(-1), which was 4% of that with L-serine as substrate. Reduction of SHMT activity in vivo was obtained by placing the essential glyA gene in the chromosome under the control of P(tac), making glyA expression isopropylthiogalactopyranoside dependent. In this way, the SHMT activity in an L-threonine producer was reduced to 8% of the initial activity, which led to a 41% reduction in glycine, while L-threonine was simultaneously increased by 49%. The intracellular availability of L-threonine to aldole cleavage was also reduced by overexpressing the L-threonine exporter thrE. In C. glutamicum DR-17, which overexpresses thrE, accumulation of 67 mM instead of 49 mM L-threonine was obtained. This shows that the potential for amino acid formation can be considerably improved by reducing its intracellular degradation and increasing its export.
Figures

References
-
- Angelaccio, S., S. Pascarella, E. Fattori, F. Bossa, W. Strong, and V. Schirch. 1992. Serine hydroxymethyltransferase: origin of substrate specificity. Biochemistry 31:155-162. - PubMed
-
- Bellmann, A., M. Vrljic, M. Pátek, H. Sahm, R. Krämer, and L. Eggeling. 2001. Regulation and specificity of the LysE-mediated export of amino acids by Corynebacterium glutamicum. Microbiology 147:1765-1774. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

