Noninvasive quantitative measurement of bacterial growth in porous media under unsaturated-flow conditions
- PMID: 12089048
- PMCID: PMC126793
- DOI: 10.1128/AEM.68.7.3597-3605.2002
Noninvasive quantitative measurement of bacterial growth in porous media under unsaturated-flow conditions
Abstract
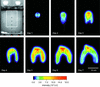
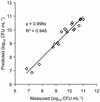
Glucose-dependent growth of the luxCDABE reporter bacterium Pseudomonas fluorescens HK44 was monitored noninvasively in quartz sand under unsaturated-flow conditions within a 45- by 56- by 1-cm two-dimensional light transmission chamber. The spatial and temporal development of growth were mapped daily over 7 days by quantifying salicylate-induced bioluminescence. A nonlinear model relating the rate of increase in light emission after salicylate exposure to microbial density successfully predicted growth over 4 orders of magnitude (r(2) = 0.95). Total model-predicted growth agreed with growth calculated from the mass balance of the system by using previously established growth parameters of HK44 (predicted, 1.2 x 10(12) cells; calculated, 1.7 x 10(12) cells). Colonization expanded in all directions from the inoculation region, including upward migration against the liquid flow. Both the daily rate of expansion of the colonized zone and the population density of the first day's growth in each newly colonized region remained relatively constant throughout the experiment. Nonetheless, substantial growth continued to occur on subsequent days in the older regions of the colonized zone. The proportion of daily potential growth that remained within the chamber declined progressively between days 2 and 7 (from 97 to 13%). A densely populated, anoxic region developed in the interior of the colonized zone even though the sand was unsaturated and fresh growth medium continued to flow through the colonized zone. These data illustrate the potential of a light transmission chamber, bioluminescent bacteria, and sensitive digital camera technology to noninvasively study real-time hydrology-microbiology interactions associated with unsaturated flow in porous media.
Figures



References
-
- Allison, D. G., B. Ruiz, C. SanJose, A. Jaspe, and P. Gilbert. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179-184. - PubMed
-
- Brink, R. H., P. Dubach, and D. L. Lynch. 1960. Measurement of carbohydrates in soil hydrolyzates with anthrone. Soil Sci. 89:157-166.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

