Multiplex, quantitative, real-time PCR assay for cytomegalovirus and human DNA
- PMID: 12089251
- PMCID: PMC120584
- DOI: 10.1128/JCM.40.7.2381-2386.2002
Multiplex, quantitative, real-time PCR assay for cytomegalovirus and human DNA
Abstract
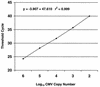
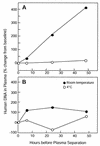
We created a multiplex, quantitative, real-time PCR assay that amplifies cytomegalovirus (CMV) and human DNA in the same reaction tube, allowing for a viral load determination that is normalized to measured human DNA. The assay targets a conserved region of the CMV DNA polymerase gene that is not affected by known drug resistance mutations. All 36 strains of CMV detected by culture or qualitative PCR in a population of lung transplant recipients were detected. The assay detected 1 to 10 copies of CMV plasmid DNA. The analytic sensitivity was not affected by the presence of DNA from 10(6) human cells but was reduced approximately 10-fold by alkaline lysates of leukocyte preparations. CMV quantitation was linear over a range of 10(1) to 10(6) copies. The intraassay and interassay coefficients of variation were 29 and 40%. Human DNA was regularly detected in patient plasma samples, and the amount was increased by storage of blood at room temperature before plasma separation and by plasma separation techniques that allowed leukocyte contamination. Applied to whole blood, the assay provides a measurement of CMV DNA in relation to cellular content without a need for cell counting procedures. Applied to plasma, the assay can reveal artifactual increases in plasma CMV levels resulting from leukocyte contamination. Further study of the utility of this assay to monitor patient populations at risk for CMV disease is warranted.
Figures


Similar articles
-
Quantitation of cytomegalovirus DNA by real-time polymerase chain reaction in peripheral blood specimens of patients with solid organ transplants: comparison with end-point PCR and pp65 antigen test.J Med Virol. 2006 Jul;78(7):915-22. doi: 10.1002/jmv.20641. J Med Virol. 2006. PMID: 16721848
-
Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR.J Clin Microbiol. 2004 Mar;42(3):1142-8. doi: 10.1128/JCM.42.3.1142-1148.2004. J Clin Microbiol. 2004. PMID: 15004066 Free PMC article.
-
Comparison of a duplex quantitative real-time PCR assay and the COBAS Amplicor CMV Monitor test for detection of cytomegalovirus.J Clin Microbiol. 2004 May;42(5):1909-14. doi: 10.1128/JCM.42.5.1909-1914.2004. J Clin Microbiol. 2004. PMID: 15131148 Free PMC article.
-
Monitoring of CMV infection: a comparison of PCR from whole blood, plasma-PCR, pp65-antigenemia and virus culture in patients after bone marrow transplantation.Bone Marrow Transplant. 1996 May;17(5):861-8. Bone Marrow Transplant. 1996. PMID: 8733710
-
Quantitation of human cytomegalovirus DNA in leukocytes by end-point titration and duplex polymerase chain reaction.J Virol Methods. 1994 Sep;49(2):195-208. doi: 10.1016/0166-0934(94)90044-2. J Virol Methods. 1994. PMID: 7822461 Clinical Trial.
Cited by
-
Assessment of Diagnostic Yield of Nonculture Infection Testing on Cerebrospinal Fluid in Immune-Competent Children.JAMA Netw Open. 2019 Jul 3;2(7):e197307. doi: 10.1001/jamanetworkopen.2019.7307. JAMA Netw Open. 2019. PMID: 31322691 Free PMC article.
-
Case Report: Approaches for managing resistant cytomegalovirus in pediatric allogeneic hematopoietic cell transplantation recipients.Front Pediatr. 2024 May 30;12:1394006. doi: 10.3389/fped.2024.1394006. eCollection 2024. Front Pediatr. 2024. PMID: 38884102 Free PMC article.
-
Safety and efficacy of prolonged cytomegalovirus prophylaxis with intravenous ganciclovir in pediatric and young adult lung transplant recipients.Pediatr Transplant. 2007 May;11(3):312-8. doi: 10.1111/j.1399-3046.2006.00626.x. Pediatr Transplant. 2007. PMID: 17430489 Free PMC article. Clinical Trial.
-
Detection of viruses in young children with fever without an apparent source.Pediatrics. 2012 Dec;130(6):e1455-62. doi: 10.1542/peds.2012-1391. Epub 2012 Nov 5. Pediatrics. 2012. PMID: 23129086 Free PMC article.
-
An inter-platform repeatability study investigating real-time amplification of plasmid DNA.BMC Biotechnol. 2005 May 25;5:15. doi: 10.1186/1472-6750-5-15. BMC Biotechnol. 2005. PMID: 15916714 Free PMC article.
References
-
- Anker, P. 2000. Quantitative aspects of plasma/serum DNA in cancer patients. Ann. N. Y. Acad. Sci. 906:5-7. - PubMed
-
- Boivin, G., R. Belanger, R. Delage, C. Beliveau, C. Demers, N. Goyette, and J. Roy. 2000. Quantitative analysis of cytomegalovirus (CMV) viremia using the pp65 antigenemia assay and the COBAS AMPLICOR CMV MONITOR PCR test after blood and marrow allogeneic transplantation. J. Clin. Microbiol. 38:4356-4360. - PMC - PubMed
-
- Boivin, G., J. Handfield, E. Toma, G. Murray, R. Lalonde, and M. G. Bergeron. 1998. Comparative evaluation of the cytomegalovirus DNA load in polymorphonuclear leukocytes and plasma of human immunodeficiency virus-infected subjects. J. Infect. Dis. 177:355-360. - PubMed
-
- Bowen, E. F., C. A. Sabin, P. Wilson, P. D. Griffiths, C. C. Davey, M. A. Johnson, and V. C. Emery. 1997. Cytomegalovirus (CMV) viraemia detected by polymerase chain reaction identifies a group of HIV-positive patients at high risk of CMV disease. AIDS 11:889-893. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

