Accuracy of results obtained by performing a second ligase chain reaction assay and PCR analysis on urine samples with positive or near-cutoff results in the LCx test for Chlamydia trachomatis
- PMID: 12089293
- PMCID: PMC120557
- DOI: 10.1128/JCM.40.7.2632-2634.2002
Accuracy of results obtained by performing a second ligase chain reaction assay and PCR analysis on urine samples with positive or near-cutoff results in the LCx test for Chlamydia trachomatis
Abstract
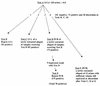
Nucleic acid amplification assays such as the ligase chain reaction and PCR have encountered reproducibility problems. The initial extract and a newly extracted aliquot of urine specimens (n = 120) which had signal-to-cutoff (S/CO) ratios above 0.80 by the LCx Chlamydia assay were retested. Nucleic acid was extracted from an additional urine sample for testing by the AMPLICOR PCR Chlamydia assay. Fifteen percent (18 of 120) of the urine specimens were negative by all repeat tests (initial mean S/CO ratio by the LCx Chlamydia assay, 0.93; S/CO ratio range, 0.80 to 3.30). Repeat testing of the 102 specimens with possible positive results by the LCx Chlamydia assay by use of the initially extracted aliquot confirmed the results for 95 (93.1%) of the specimens; repeat testing of a newly extracted aliquot confirmed the results for 87 (85.3%) of the specimens. Twenty specimens had discordant results by the two repeat LCx Chlamydia assays. A total of 78 of 102 (76.5%) of the specimens were positive by the AMPLICOR PCR, and the AMPLICOR PCR confirmed the results for 82.1% (78 of 95) and 89.6% (78 of 87) of the specimens positive by the two repeat LCx Chlamydia assays, respectively. Some of the discrepancies observed by multiple repeat tests may have been due to specimen mislabeling or contamination during performance of the procedure rather than to the LCx Chlamydia assay. Both assays suffered from a lack of reproducibility on repeat testing with a small proportion of specimens, probably due to the presence of low levels of DNA, the presence of variable amounts of amplification inhibitors, and the loss of DNA during extraction.
Figures
References
-
- Coutlee, F., M. de Ladurantaye, C. Tremblay, J. Vincelette, L. Labrecque, and M. Roger. 2000. An important proportion of genital samples submitted for Chlamydia trachomatis detection by PCR contain small amounts of cellular DNA as measured by β-globin gene amplification. J. Clin. Microbiol. 38:2512-2515. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical