The function of the Periaxin gene during nerve repair in a model of CMT4F
- PMID: 12090399
- PMCID: PMC1570694
- DOI: 10.1046/j.1469-7580.2002.00038.x
The function of the Periaxin gene during nerve repair in a model of CMT4F
Abstract
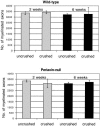
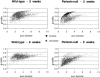
Mutations in the Periaxin (PRX) gene are known to cause autosomal recessive demyelinating Charcot-Marie-Tooth (CMT4F) and Dejerine-Sottas disease. The pathogenesis of these diseases is not fully understood. However, progress is being made by studying both the periaxin-null mouse, a mouse model of the disease, and the protein-protein interactions of periaxin. L-periaxin is a constituent of the dystroglycan-dystrophin-related protein-2 complex linking the Schwann cell cytoskeleton to the extracellular matrix. Although periaxin-null mice myelinate normally, they develop a demyelinating peripheral neuropathy later in life. This suggests that periaxin is required for the stable maintenance of a normal myelin sheath. We carried out sciatic nerve crushes in 6-week-old periaxin-null mice, and, 6 weeks later, found that although the number of myelinated axons had returned to normal, the axon diameters remained smaller than in the contralateral uncrushed nerve. Not only do periaxin-null mice have more hyper-myelinated axons than their wild-type counterparts but they also recapitulate this hypermyelination during regeneration. Therefore, periaxin-null mice can undergo peripheral nerve remyelination, but the regulation of peripheral myelin thickness is disrupted.
Figures




References
-
- Bolino A, Muglia M, Conforti FL, LeGuern E, Salih MA, Georgiou DM, et al. Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat. Genet. 2000;25:17–19. - PubMed
-
- Bridge PM, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA, et al. Nerve crush injuries – a model for axonotmesis. Exp. Neurol. 1994;127:284–290. - PubMed
-
- Dyck PJ, Lambert EH. Lower motor and primary sensory neuron diseases with peroneal muscular atrophy. I. Neurologic, genetic, and electrophysiologic findings in hereditary polyneuropathies. Arch. Neurol. 1968;18:603–618. - PubMed
-
- Dytrych L, Sherman DL, Gillespie CS, Brophy PJ. Two PDZ domain proteins encoded by the murine periaxin gene are the result of alternative intron retention and are differentially targeted in Schwann cells. J. Biol. Chem. 1998;273:5794–5800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

