Role of immune cells in animal models for inherited neuropathies: facts and visions
- PMID: 12090406
- PMCID: PMC1570697
- DOI: 10.1046/j.1469-7580.2002.00045.x
Role of immune cells in animal models for inherited neuropathies: facts and visions
Abstract
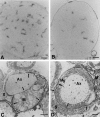
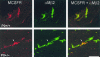
Mice heterozygously deficient in the peripheral myelin adhesion molecule P0 (P0+/- mice) are models for some forms of Charcot-Marie-Tooth (CMT) neuropathies. In addition to the characteristic hallmarks of demyelination, elevated numbers of CD8-positive T-lymphocytes and F4/80-positive macrophages are striking features in the nerves of these mice. These immune cells increase in number with age and progress of demyelination, suggesting that they might be functionally related to myelin damage. In order to investigate the pathogenetic role of lymphocytes, the myelin mutants were cross-bred with recombination activating gene 1 (RAG-1)-deficient mice, which lack mature T- and B-lymphocytes. The immunodeficient myelin mutants showed a less severe myelin degeneration. The beneficial effect of lymphocyte-deficiency was reversible, since demyelination worsened in immunodeficient myelin-mutants when reconstituted with bone marrow from wild-type mice. Ultrastructural analysis revealed macrophages in close apposition to myelin and demyelinated axons. We therefore cross-bred the P0+/- mice with spontaneous osteopetrotic (op) mutants deficient in the macrophage colony-stimulating factor (M-CSF), hence displaying impaired macrophage activation. In the corresponding double mutants the numbers of macrophages were not elevated in the peripheral nerves, and the demyelinating phenotype was less severe than in the genuine P0+/- mice, demonstrating that macrophages are also functionally involved in the pathogenesis of genetically mediated demyelination. We also examined other models for inherited neuropathies for a possible involvement of immune cells. We chose mice deficient in the gap junction component connexin 32, a model for the X-linked form of CMT. Similar to P0-deficient mice, T-lymphocytes and macrophages were elevated and macrophages showed a close apposition to degenerating myelin. We conclude that the involvement of T-lymphocytes and macrophages is a common pathogenetic feature in various forms of slowly progressive inherited neuropathies.
Figures



References
-
- Abrams CK, Oh S, Ri Y, Bargiello TA. Mutations in connexin 32: the molecular and biophysical bases for the X-linked form of Charcot-Marie-Tooth disease. Brain Res. Rev. 2000;32:203–214. - PubMed
-
- Anderson TJ, Schneider A, Barrie BA, Klugmann MMC, Culloch MC, Kirkham D, et al. Late-onset neurodegeneration in mice with increased dosage of the proteolipid protein gene. J. Comp. Neurol. 1998;394:506–519. - PubMed
-
- Ballin RH, Thomas PK. Electron microscope observations on demyelination and remyelination in experimental allergic neuritis I Demyelination. J. Neurol. Sci. 1969;8:1–18. - PubMed
-
- Berger J, Moser HW, Forss-Petter S. Leukodystrophies: recent developments in genetics, molecular biology, pathogenesis and treatment. Curr. Opin. Neurol. 2001;14:305–312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

