Axons and glial interfaces: ultrastructural studies
- PMID: 12090407
- PMCID: PMC1570690
- DOI: 10.1046/j.1469-7580.2002.00037.x
Axons and glial interfaces: ultrastructural studies
Abstract
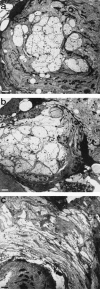
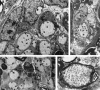
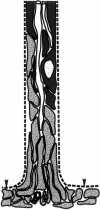
At most vertebrate nerve transitional zones (TZs) there is a glial barrier which is pierced by axons passing between the CNS and PNS. Myelinated axons traverse this in individual tunnels. The same is true of larger non-myelinated axons. This holds widely among the vertebrates, for example, the large motor axons of the sea-lamprey Petromyzon (which also possess TZ specializations not found in mammals). Smaller non-myelinated axons traverse the TZ glial tunnels as fascicles and so the barriers are correspondingly less comprehensive for them. Accordingly, in nerves composed of non-myelinated axons, such as the vomeronasal or the olfactory, a TZ barrier stretching across the nerve is effectivelyabsent. The chordateAmphioxus differsfrom the vertebrates in lacking a TZ barrier throughout. Invertebrates also lack glial barriers at the TZs between ganglia and interconnecting nerve trunks. The glial barrier at the dorsal spinal root TZ (DRTZ) has considerable value for analysing protocols aimed at achieving CNS regeneration, because it provides a useful model of the gliotic reaction at sites of CNS injury. Also, it is especially amenable to morphometric analysis, and so enables objective quantification of different protocols. Being adjacent to the subarachnoid space, it is accessible for experimental intervention. The DRTZ was used to investigate the value of neurotrophin 3 (NT3) in promoting axon regeneration across the TZ barrier and into the CNS following dorsal root crush. It promoted extensive regeneration and vigorous non-myelinated axonal ensheathment. On average, around 40% of regenerating axons grew across the interface, compared with virtually none in its absence. These may have traversed the interface through loci occupied by axons prior to degeneration. Many regenerating axons became myelinated, both centrally and peripherally.
Figures










References
-
- Berthold C-H, Carlstedt T, Corneliuson O. Anatomy of the nerve root at the central-peripheral transitional region. In: Dyck PJ, Thomas PK, Lambert EH, Bunge R, editors. Peripheral Neuropathy. Vol. 1. Philadelphia: W.B. Saunders; 1984. pp. 156–170.
-
- Bovolenta P, Wandosell F, Nieto-Sampedro M. CNS glial scar tissue: a source of molecules which inhibit central neurite outgrowth. In: Yu ACH, Hertz L, Norneberg MD, Sykova E, Waxman SG, editors. Progress in Brain Research. Vol. 94. Netherlands: Elsevier Science; 1992. pp. 367–379. - PubMed
-
- Bovolenta P, Wandosell F, Nieto-Sampedro M. Neurite outgrowth inhibitors associated with glial cells and glial cell lines. Neuroreport. 1993;5:345–348. - PubMed
-
- Carlstedt T. Regenerating axons form nerve terminals at astrocytes. Brain. Res. 1985a;347:188–191. - PubMed
-
- Carlstedt T. Dorsal root innervation of spinal cord neurons after dorsal root implantation into the spinal cord of adult rats. Neurosci. Lett. 1985b;55:343–348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

