Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment
- PMID: 12091880
- PMCID: PMC2779715
- DOI: 10.1038/nm740
Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment
Abstract
The mechanism by which angiogenic factors recruit bone marrow (BM)-derived quiescent endothelial and hematopoietic stem cells (HSCs) is not known. Here, we report that functional vascular endothelial growth factor receptor-1 (VEGFR1) is expressed on human CD34(+) and mouse Lin(-)Sca-1(+)c-Kit(+) BM-repopulating stem cells, conveying signals for recruitment of HSCs and reconstitution of hematopoiesis. Inhibition of VEGFR1, but not VEGFR2, blocked HSC cell cycling, differentiation and hematopoietic recovery after BM suppression, resulting in the demise of the treated mice. Placental growth factor (PlGF), which signals through VEGFR1, restored early and late phases of hematopoiesis following BM suppression. PlGF enhanced early phases of BM recovery directly through rapid chemotaxis of VEGFR1(+) BM-repopulating and progenitor cells. The late phase of hematopoietic recovery was driven by PlGF-induced upregulation of matrix metalloproteinase-9, mediating the release of soluble Kit ligand. Thus, PlGF promotes recruitment of VEGFR1(+) HSCs from a quiescent to a proliferative BM microenvironment, favoring differentiation, mobilization and reconstitution of hematopoiesis.
Conflict of interest statement
Competing interests statement
The authors declare competing financial interests: see the website (
Figures

 , 1 × 105;
, 1 × 105;  , 1 × 103;
, 1 × 103;  , 1 × 102;
, 1 × 102;  , 1 × 101 VEGFR1+ BMMCs. ○, 1 × 105 VEGFR1− BMMCs.
, 1 × 101 VEGFR1+ BMMCs. ○, 1 × 105 VEGFR1− BMMCs.  , 1 × 105 VEGFR2+ BMMCs. Mice transplanted with 1 × 102 VEGFR1+ BMMCs showed improved survival versus mice transplanted with 1 × 105 VEGFR1− BMMCs or VEGFR2+ BMMCs (n = 10; *, P < 0.05). d and e, BM cells obtained from 5FU-treated C57BL/6-Ly5.2 mice were stained for VEGFR1-Cy2 and Sca-1-PE. Singly and doubly positive VEGFR1/Sca-1 BM cells (1 × 103) isolated by MoFlo cell sorter were transplanted into lethally irradiated C57BL/6-Ly5.1 mice. ■, VEGFR1+Sca-1+;
, 1 × 105 VEGFR2+ BMMCs. Mice transplanted with 1 × 102 VEGFR1+ BMMCs showed improved survival versus mice transplanted with 1 × 105 VEGFR1− BMMCs or VEGFR2+ BMMCs (n = 10; *, P < 0.05). d and e, BM cells obtained from 5FU-treated C57BL/6-Ly5.2 mice were stained for VEGFR1-Cy2 and Sca-1-PE. Singly and doubly positive VEGFR1/Sca-1 BM cells (1 × 103) isolated by MoFlo cell sorter were transplanted into lethally irradiated C57BL/6-Ly5.1 mice. ■, VEGFR1+Sca-1+;  , VEGFR1+Sca-1−;
, VEGFR1+Sca-1−;  , VEGFR1−Sca-1+;
, VEGFR1−Sca-1+;  , VEGFR1−Sca-1− of BM cells. d, Survival rate was improved in mice transplanted with 1 × 103 VEGFR1+Sca-1− cells compared with mice transplanted with VEGFR1−Sca-1+ cells (n = 12; *, P < 0.05). e, 5 mo after transplantation, the percentage of donor (Ly5.2)-derived myeloid (CD11b and Gr-1) and lymphoid (Thy-1 and B220) cells in the peripheral blood of recipient mice (Ly5.1) was analyzed by FACS. A representative FACS analysis is shown in which 88.3% chimerism was achieved 150 d after transplantation.
, VEGFR1−Sca-1− of BM cells. d, Survival rate was improved in mice transplanted with 1 × 103 VEGFR1+Sca-1− cells compared with mice transplanted with VEGFR1−Sca-1+ cells (n = 12; *, P < 0.05). e, 5 mo after transplantation, the percentage of donor (Ly5.2)-derived myeloid (CD11b and Gr-1) and lymphoid (Thy-1 and B220) cells in the peripheral blood of recipient mice (Ly5.1) was analyzed by FACS. A representative FACS analysis is shown in which 88.3% chimerism was achieved 150 d after transplantation.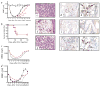
 ), anti-VEGFR2 (●) or IgG control (○) in 2-d intervals starting from day 0. Mice were also treated with anti-VEGFR1 (□) and anti-VEGFR2 (◇) alone. a, WBCs were quantified. b, Survival rate was monitored on a daily basis. Survival rate was lower in the VEGFR1-treated versus IgG-treated mice (*, P < 0.001). c and d, Hematopoietic recovery following a single dose of 5FU on day 0 (c) or total body irradiation (TBI) plus carboplatin at day 0 (d) (n = 6 for both) followed by a single dose of either Ad-PlGF (
), anti-VEGFR2 (●) or IgG control (○) in 2-d intervals starting from day 0. Mice were also treated with anti-VEGFR1 (□) and anti-VEGFR2 (◇) alone. a, WBCs were quantified. b, Survival rate was monitored on a daily basis. Survival rate was lower in the VEGFR1-treated versus IgG-treated mice (*, P < 0.001). c and d, Hematopoietic recovery following a single dose of 5FU on day 0 (c) or total body irradiation (TBI) plus carboplatin at day 0 (d) (n = 6 for both) followed by a single dose of either Ad-PlGF ( ) or Ad-null (○) on day 0. d, Recombinant G-CSF (injected subcutaneously day 0–14, ■) promotes rapid hematopoietic reconstitution. Extent and duration of less than 2000/μl WBCs was significantly shorter in the Ad-PlGF-treated versus Ad-null-treated 5FU- and carboplatin/irradiation-treated mice. c, *, P < 0.001; d, *, P < 0.005. Error bars represent mean ± s.e.m. for 6–10 mice per group. e–g, H&E staining of BM sections of 5FU-treated mice 10 d after co-injection with neutralizing anti-VEGFR1 (f), anti-VEGFR2 (g) or IgG controls (e). Magnification, × 100. h–m, vWF staining of BM sections 10 d after 5FU treatment in antibody-treated mice (anti-VEGFR1 (i and l), anti-VEGFR2 (j and m) or IgG controls (h and k); megakaryocytes are depicted by arrows). Magnifications, ×100 (h–j); ×400 (k–m).
) or Ad-null (○) on day 0. d, Recombinant G-CSF (injected subcutaneously day 0–14, ■) promotes rapid hematopoietic reconstitution. Extent and duration of less than 2000/μl WBCs was significantly shorter in the Ad-PlGF-treated versus Ad-null-treated 5FU- and carboplatin/irradiation-treated mice. c, *, P < 0.001; d, *, P < 0.005. Error bars represent mean ± s.e.m. for 6–10 mice per group. e–g, H&E staining of BM sections of 5FU-treated mice 10 d after co-injection with neutralizing anti-VEGFR1 (f), anti-VEGFR2 (g) or IgG controls (e). Magnification, × 100. h–m, vWF staining of BM sections 10 d after 5FU treatment in antibody-treated mice (anti-VEGFR1 (i and l), anti-VEGFR2 (j and m) or IgG controls (h and k); megakaryocytes are depicted by arrows). Magnifications, ×100 (h–j); ×400 (k–m).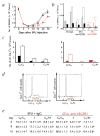
 ), VEGFR2 (●) or IgG controls (○) in 2-day intervals. 3 mice treated with anti-VEGFR1 or anti-IgG were killed at each time point. Quantification of total BMMCs obtained from BM of treated and untreated mice (*, P < 0.05; **, P < 0.01). b, BMMCs were stained with different lineage-restricted antigens including the B cell–associated antigen B220 (shaded bar), the myeloid marker CD11b (empty bar) and the erythroid marker TER119 (filled bar). *, P < 0.05 for absolute number of lineage-derived cells in the anti-VEGFR1-treated versus the control groups. c, Cell cycle of Lin−Sca-1+VEGFR1+ cells, after 5FU treatment, was determined after staining of Lin−Sca-1+ cells with VEGFR1-Cy2 and propidium iodide on day 6 (■) and day 10 (□) after 5FU + IgG (left) and 5FU + anti-VEGFR1(right). DNA content was quantified and assessed by flow cytometry. Absolute number of cells in different phases of the cell cycle is given. d and e, Cell cycle of Sca-1+ BMMCs, after 5FU treatment, was determined by staining with Sca-1-FITC and propidium iodide. Representative data from day 6 are shown as histograms with IgG (left) and anti-VEGFR1 (right) (d). Percentage of Sca-1+ cells in the S and G2/M phase from anti-VEGFR1-treated versus IgG-treated control group (e). *, P < 0.001. Error bars represent mean ± s.e.m. for 9 mice per group (a) or for 3 independent experiments (b, c, and e).
), VEGFR2 (●) or IgG controls (○) in 2-day intervals. 3 mice treated with anti-VEGFR1 or anti-IgG were killed at each time point. Quantification of total BMMCs obtained from BM of treated and untreated mice (*, P < 0.05; **, P < 0.01). b, BMMCs were stained with different lineage-restricted antigens including the B cell–associated antigen B220 (shaded bar), the myeloid marker CD11b (empty bar) and the erythroid marker TER119 (filled bar). *, P < 0.05 for absolute number of lineage-derived cells in the anti-VEGFR1-treated versus the control groups. c, Cell cycle of Lin−Sca-1+VEGFR1+ cells, after 5FU treatment, was determined after staining of Lin−Sca-1+ cells with VEGFR1-Cy2 and propidium iodide on day 6 (■) and day 10 (□) after 5FU + IgG (left) and 5FU + anti-VEGFR1(right). DNA content was quantified and assessed by flow cytometry. Absolute number of cells in different phases of the cell cycle is given. d and e, Cell cycle of Sca-1+ BMMCs, after 5FU treatment, was determined by staining with Sca-1-FITC and propidium iodide. Representative data from day 6 are shown as histograms with IgG (left) and anti-VEGFR1 (right) (d). Percentage of Sca-1+ cells in the S and G2/M phase from anti-VEGFR1-treated versus IgG-treated control group (e). *, P < 0.001. Error bars represent mean ± s.e.m. for 9 mice per group (a) or for 3 independent experiments (b, c, and e).
 , WBCs in Ad-PlGF-treated group; ■, WBCs in Ad-null-treated group;
, WBCs in Ad-PlGF-treated group; ■, WBCs in Ad-null-treated group;  , neutrophils in Ad-PlGF-treated group; (□), neutrophils in Ad-null-treated group. Plasma PlGF levels after adenovirus administration were measured by ELISA (insert). b, Number of mobilized progenitors was quantified by using CFU assays. As compared with Ad-null controls, there was a 14-fold increase in the number of circulating progenitors in Ad-PlGF-treated mice. □, CFU-GM; ■, CFU-GEMM;
, neutrophils in Ad-PlGF-treated group; (□), neutrophils in Ad-null-treated group. Plasma PlGF levels after adenovirus administration were measured by ELISA (insert). b, Number of mobilized progenitors was quantified by using CFU assays. As compared with Ad-null controls, there was a 14-fold increase in the number of circulating progenitors in Ad-PlGF-treated mice. □, CFU-GM; ■, CFU-GEMM;  , CFU-M;
, CFU-M;  , BFU-E. (n = 6; *, P < 0.001). c and d, Pluripotency of mobilized cells was assessed by CFU-S assay (c) and BM repopulating assay (d). c, Mobilized PBMCs from Ad-PlGF (
, BFU-E. (n = 6; *, P < 0.001). c and d, Pluripotency of mobilized cells was assessed by CFU-S assay (c) and BM repopulating assay (d). c, Mobilized PBMCs from Ad-PlGF ( ), Ad-null (□) and control mice (■) were injected into lethally irradiated recipient mice and spleens were harvested on day 12. CFU-S were counted (*, P < 0.001). d, PBMCs obtained from male donor mice were collected 1 d after treatment with Ad-null and Ad-PlGF vectors. PBMCs of Ad-PlGF-treated mice (◇, 1 × 106;
), Ad-null (□) and control mice (■) were injected into lethally irradiated recipient mice and spleens were harvested on day 12. CFU-S were counted (*, P < 0.001). d, PBMCs obtained from male donor mice were collected 1 d after treatment with Ad-null and Ad-PlGF vectors. PBMCs of Ad-PlGF-treated mice (◇, 1 × 106;  , 5 × 105;
, 5 × 105;  , 1 × 105;
, 1 × 105;  , 5 × 104 cells) and Ad-null-treated mice were transplanted into lethally irradiated female recipients (n = 12 per group). In mice injected with as low as 1 × 105 PlGF-mobilized PBMC cells, there was a significant improvement in survival of recipient mice versus mice transplanted with Ad-null-derived PBMCs (*, P < 0.05). e, Human CD34+ cells were plated in Matrigel-coated transwells. The chemoat-tractants PlGF or VEGF were added to the lower chamber, while anti-VEGFR1 was added to both chambers. Data are shown as a percentage of migrated cells (□). Migrated cells with stem cell potential were quantified by the capacity of cobblestone formation on wk 5, (CAFC wk 5; ■) (n = 3; *, P < 0.001 for migration towards chemoattractant PlGF of cells treated with/without anti-VEGFR1). Bottom, Gelatin zymogram of supernatants from human CD34+ cells stimulated with or without PlGF or VEGF in serum-free medium. Supernatants from CD34+-cell cultures showed gelatinolytic activity for pro-MMP-9 (92 kD). Error bars represent mean ± s.e.m. for 6 mice per experimental condition (a), and 3 independent experiments (b, c, e).
, 5 × 104 cells) and Ad-null-treated mice were transplanted into lethally irradiated female recipients (n = 12 per group). In mice injected with as low as 1 × 105 PlGF-mobilized PBMC cells, there was a significant improvement in survival of recipient mice versus mice transplanted with Ad-null-derived PBMCs (*, P < 0.05). e, Human CD34+ cells were plated in Matrigel-coated transwells. The chemoat-tractants PlGF or VEGF were added to the lower chamber, while anti-VEGFR1 was added to both chambers. Data are shown as a percentage of migrated cells (□). Migrated cells with stem cell potential were quantified by the capacity of cobblestone formation on wk 5, (CAFC wk 5; ■) (n = 3; *, P < 0.001 for migration towards chemoattractant PlGF of cells treated with/without anti-VEGFR1). Bottom, Gelatin zymogram of supernatants from human CD34+ cells stimulated with or without PlGF or VEGF in serum-free medium. Supernatants from CD34+-cell cultures showed gelatinolytic activity for pro-MMP-9 (92 kD). Error bars represent mean ± s.e.m. for 6 mice per experimental condition (a), and 3 independent experiments (b, c, e).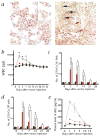
 , Ad-PlGF in MMP-9−/−; ●, Ad-null in MMP-9−/− mice; *, P < 0.001). c, Mobilized PBMCs were plated in a colony assay (*, P < 0.01). d, Mobilized PBMCs were injected into lethally irradiated mice and spleen colony formation (CFU-S) was determined (*, P < 0.01; black bar: Ad-null in wild-type mice, red bar: Ad-PlGF in MMP-9−/− mice, open bar: Ad-PlGF in wild-type mice). e, Plasma levels of sKitL in MMP-9−/− and MMP-9+/+ mice were determined by ELISA. □, Ad-PlGF in wild type;
, Ad-PlGF in MMP-9−/−; ●, Ad-null in MMP-9−/− mice; *, P < 0.001). c, Mobilized PBMCs were plated in a colony assay (*, P < 0.01). d, Mobilized PBMCs were injected into lethally irradiated mice and spleen colony formation (CFU-S) was determined (*, P < 0.01; black bar: Ad-null in wild-type mice, red bar: Ad-PlGF in MMP-9−/− mice, open bar: Ad-PlGF in wild-type mice). e, Plasma levels of sKitL in MMP-9−/− and MMP-9+/+ mice were determined by ELISA. □, Ad-PlGF in wild type;  , Ad-PlGF in MMP-9−/− mice; ■, Ad-null in wild-type mice; ●, Ad-null in MMP-9−/− mice. Error bars represent mean ± s.e.m. for 6 mice per group (b) and for 3 separate experiments per variable (c, d and e)
, Ad-PlGF in MMP-9−/− mice; ■, Ad-null in wild-type mice; ●, Ad-null in MMP-9−/− mice. Error bars represent mean ± s.e.m. for 6 mice per group (b) and for 3 separate experiments per variable (c, d and e)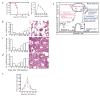
 , 5FU+anti-VEGFR1. Right, Plasma levels of sKitL were determined by ELISA. ○, AdsKitL; ■, Ad-null. b–d, BMMCs were counted and H&E staining of femurs of anti-VEGFR1 (b), anti-VEGFR1+AdsKitL (c) and AdsKitL-treated (d) mice was performed for morphological analysis, magnification, ×400. *, P < 0.01 comparing the absolute number of BMMCs in the anti-VEGFR1 vs. the control groups. e, Plasma sKitL levels were measured by ELISA in 5FU-treated mice co-injected with neutralizing doses of mAb to VEGFR1 or IgG (n = 5; *, P < 0.01 comparing sKitL plasma levels in mice treated with anti-VEGFR1 to controls). ○, 5FU+IgG;
, 5FU+anti-VEGFR1. Right, Plasma levels of sKitL were determined by ELISA. ○, AdsKitL; ■, Ad-null. b–d, BMMCs were counted and H&E staining of femurs of anti-VEGFR1 (b), anti-VEGFR1+AdsKitL (c) and AdsKitL-treated (d) mice was performed for morphological analysis, magnification, ×400. *, P < 0.01 comparing the absolute number of BMMCs in the anti-VEGFR1 vs. the control groups. e, Plasma sKitL levels were measured by ELISA in 5FU-treated mice co-injected with neutralizing doses of mAb to VEGFR1 or IgG (n = 5; *, P < 0.01 comparing sKitL plasma levels in mice treated with anti-VEGFR1 to controls). ○, 5FU+IgG;  , 5FU+anti-VEGFR1; □, 5FU+anti-VEGFR2. f, PlGF mediates the early phase of BM recovery through rapid mobilization/chemotaxis of preexisting VEGFR1+ BM-repopulating cells. During the late phase of BM recovery PlGF promotes hematopoiesis primarily through MMP-9 mediated release of sKitL, resulting in enhanced cell motility, cycling and differentiation of VEGFR1+ long-term repopulating cells. Error bars represent mean ± s.e.m. for 2–-5 separate experiments (a–e).
, 5FU+anti-VEGFR1; □, 5FU+anti-VEGFR2. f, PlGF mediates the early phase of BM recovery through rapid mobilization/chemotaxis of preexisting VEGFR1+ BM-repopulating cells. During the late phase of BM recovery PlGF promotes hematopoiesis primarily through MMP-9 mediated release of sKitL, resulting in enhanced cell motility, cycling and differentiation of VEGFR1+ long-term repopulating cells. Error bars represent mean ± s.e.m. for 2–-5 separate experiments (a–e).Comment in
-
VEGF receptor 1 stimulates stem-cell recruitment and new hope for angiogenesis therapies.Nat Med. 2002 Aug;8(8):775-7. doi: 10.1038/nm0802-775. Nat Med. 2002. PMID: 12152025 No abstract available.
References
-
- Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature Med. 2001;7:1194–1201. - PubMed
-
- Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. - PubMed
-
- Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–851. - PubMed
-
- Berardi AC, Wang A, Levine JD, Lopez P, Scadden DT. Functional isolation and characterization of human hematopoietic stem cells. Science. 1995;267:104–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS039278/NS/NINDS NIH HHS/United States
- R01 HL-66592/HL/NHLBI NIH HHS/United States
- P01 HL067839/HL/NHLBI NIH HHS/United States
- R01 HL-58707/HL/NHLBI NIH HHS/United States
- CA 72006/CA/NCI NIH HHS/United States
- AR46238/AR/NIAMS NIH HHS/United States
- R01 HL61401/HL/NHLBI NIH HHS/United States
- R01 HL061849/HL/NHLBI NIH HHS/United States
- CA 75072/CA/NCI NIH HHS/United States
- R01 HL-67839/HL/NHLBI NIH HHS/United States
- R01 CA075072/CA/NCI NIH HHS/United States
- R01 AR046238/AR/NIAMS NIH HHS/United States
- NS39278/NS/NINDS NIH HHS/United States
- P01 CA072006/CA/NCI NIH HHS/United States
- R01 HL61849/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

