Evaluation of urine as a clinical specimen for diagnosis of hepatitis a
- PMID: 12093683
- PMCID: PMC120033
- DOI: 10.1128/cdli.9.4.840-845.2002
Evaluation of urine as a clinical specimen for diagnosis of hepatitis a
Abstract
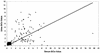
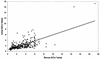
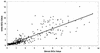
The present study pertains to the evaluation of urine as a specimen for detection of anti-hepatitis A virus (anti-HAV) antibodies. Immunoglobulin M (IgM), IgG, and IgA capture enzyme-linked immunosorbent assays for hepatitis A were performed on paired serum and urine specimens collected from hepatitis A patients (n = 92), healthy individuals (n = 100), non-A hepatitis patients (n = 70), and patients with nonhepatic diseases (n = 64, including 37 renal disease patients). Hepatitis A patients seropositive for anti-HAV IgM showed 95.65% uropositivity. No false-positive reactions were observed in control groups. The uropositivity of anti-HAV IgM persisted during the convalescent phase of the disease. Anti-HAV IgG uropositivity correlated well with corresponding seropositivity in all groups (P > 0.05 for each). No significant difference between the proportions of serum and urine positivity for anti-HAV IgA was noted (P > 0.05 for each). Using seroreactivity as a "gold standard," the sensitivity and specificity for anti-HAV IgM, anti-HAV IgG, and anti-HAV IgA tests with urine as a specimen were found to be 95.65 and 100%, 97.76 and 76.47%, and 92.23 and 88.18%, respectively. Urine appears to be comparable to serum for diagnosis of recent and past infection with hepatitis A.
Figures
References
-
- Arankalle, V. A., M. S., Chadha, S. D. Chitambar, A. M., Walimbe, L. P. Chobe, and S. S. Gandhe. 2001. Changing epidemiology of hepatitis A and E in urban and rural India. J. Viral Hepatitis 8:293-303. - PubMed
-
- Arslan, M., R. H. Wiesner, J. J. Poterucha, J. B. Gross, and N. N. Zein. 1998. Hepatitis A virus antibodies in liver transplant (OLT) recipients: evidence for loss of immunity post transplantation. Hepatology 28:235A. - PubMed
-
- Carli, K. T., H. Batmaz., A. Sen, and A. Minbay. 1993. Comparison of serum, milk and urine as samples in an enzyme immunoassay for bovine leukaemia virus infection. Res. Vet. Sci. 55:394-395. - PubMed
-
- Chio, F., and A. A. Bakir. 1992. Acute renal failure in hepatitis A. Int. J. Artif. Organs 1S:413-416. - PubMed
-
- Chitambar, S. D., S. Murthy-Grewal, M. Bokil, V. A. Arankalle, M. M. Gore, and K. Banerjee. 1994. Indigenous anti hepatitis A virus IgM capture ELISA for the diagnosis of hepatitis A. Indian J. Med. Res. 99:243-251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

