Identification, molecular cloning, and evaluation of potential use of isocitrate dehydrogenase II of Mycobacterium bovis BCG in serodiagnosis of tuberculosis
- PMID: 12093684
- PMCID: PMC120012
- DOI: 10.1128/cdli.9.4.846-851.2002
Identification, molecular cloning, and evaluation of potential use of isocitrate dehydrogenase II of Mycobacterium bovis BCG in serodiagnosis of tuberculosis
Abstract
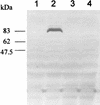
Diagnosis of tuberculosis is time-consuming and requires infrastructures which are often not available in countries with high incidences of the disease. In the present study, an 82-kDa protein antigen was isolated by affinity chromatography and was identified by peptide mass fingerprinting as isocitrate dehydrogenase II, which is encoded by the icd2 gene of Mycobacterium bovis BCG. The icd2 gene of BCG was cloned by PCR, and the product of recombinant gene expression was purified and analyzed by two-dimensional polyacrylamide gel electrophoresis. The recombinant protein, named rICD2, was tested for its recognition by immunoglobulin G (IgG) antibodies from the sera of 16 patients with tuberculosis (TB) and 23 healthy individuals by Western blotting. The results showed that rICD2 is recognized by IgG antibodies from the sera of all TB patients tested at serum dilutions of > or = 1:640. At a serum dilution of 1:1,280, the sensitivity was 50% and the specificity was 86.9%. These results indicate that rICD2 might represent a candidate for use in a new assay for the serodiagnosis of TB.
Figures




Similar articles
-
Cloning and expression of recombinant MPB70 protein antigen from Mycobacterium bovis BCG for diagnosis of tuberculosis.Egypt J Immunol. 2004;11(2):21-9. Egypt J Immunol. 2004. PMID: 16734114
-
Clinical evaluation of serodiagnosis of active tuberculosis by multiple-antigen ELISA using lipids from Mycobacterium bovis BCG Tokyo 172.Clin Chem Lab Med. 2005;43(11):1253-62. doi: 10.1515/CCLM.2005.216. Clin Chem Lab Med. 2005. PMID: 16232093
-
Enhanced patient serum immunoreactivity to recombinant Mycobacterium tuberculosis CFP32 produced in the yeast Pichia pastoris compared to Escherichia coli and its potential for serodiagnosis of tuberculosis.J Clin Microbiol. 2006 Sep;44(9):3086-93. doi: 10.1128/JCM.02672-05. J Clin Microbiol. 2006. PMID: 16954231 Free PMC article.
-
Serological response in leprosy and tuberculosis patients to the 18-kDa antigen of Mycobacterium leprae and antigen 85B of Mycobacterium bovis BCG.Int J Lepr Other Mycobact Dis. 1993 Dec;61(4):571-80. Int J Lepr Other Mycobact Dis. 1993. PMID: 8151188
-
Cloning and characterization of Clp protease proteolytic subunit 2 and its implication in clinical diagnosis of tuberculosis.Int J Clin Exp Pathol. 2014 Aug 15;7(9):5674-82. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337208 Free PMC article.
Cited by
-
Mycobacterium tuberculosis (Mtb) isocitrate dehydrogenases show strong B cell response and distinguish vaccinated controls from TB patients.Proc Natl Acad Sci U S A. 2004 Aug 24;101(34):12652-7. doi: 10.1073/pnas.0404347101. Epub 2004 Aug 16. Proc Natl Acad Sci U S A. 2004. PMID: 15314217 Free PMC article.
-
Variant tricarboxylic acid cycle in Mycobacterium tuberculosis: identification of alpha-ketoglutarate decarboxylase.Proc Natl Acad Sci U S A. 2005 Jul 26;102(30):10670-5. doi: 10.1073/pnas.0501605102. Epub 2005 Jul 18. Proc Natl Acad Sci U S A. 2005. PMID: 16027371 Free PMC article.
-
Diagnosis of tuberculosis: available technologies, limitations, and possibilities.J Clin Lab Anal. 2003;17(5):155-63. doi: 10.1002/jcla.10086. J Clin Lab Anal. 2003. PMID: 12938143 Free PMC article. Review.
-
PPE antigen Rv2430c of Mycobacterium tuberculosis induces a strong B-cell response.Infect Immun. 2003 Nov;71(11):6338-43. doi: 10.1128/IAI.71.11.6338-6343.2003. Infect Immun. 2003. PMID: 14573653 Free PMC article.
-
Identification of Missing Carbon Fixation Enzymes as Potential Drug Targets in Mycobacterium Tuberculosis.J Integr Bioinform. 2018 Jul 3;15(3):20170041. doi: 10.1515/jib-2017-0041. J Integr Bioinform. 2018. PMID: 30218604 Free PMC article.
References
-
- Chang, Z., A. Choudhary, R. Lathigra, and F. A. Quiocho. 1994. The immunodominant 38-kDa lipoprotein antigen of Mycobacterium tuberculosis is a phosphate-binding protein. J. Biol. Chem. 269:1956-1958. - PubMed
-
- Chung, A. E., and J. S. Franzen. 1969. Oxidized triphosphopiridine nucleotide specific isocitrate dehydrogenase from Azotobacter vinelandii: isolation and characterization. Biochemistry 8:3175-3184. - PubMed
-
- Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Conner, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. - PubMed
-
- Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. W. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

