Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled
- PMID: 12093741
- PMCID: PMC125394
- DOI: 10.1093/emboj/cdf331
Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled
Abstract
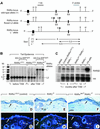
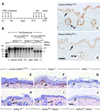
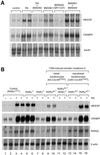
To investigate the roles of retinoic acid (RA) receptors (RARs) in the physiology of epidermis that does not express RAR beta, conditional spatio-temporally controlled somatic mutagenesis was used to selectively ablate RAR alpha in keratinocytes of RAR gamma-null mice. Keratinocyte proliferation was maintained in adult mouse epidermis lacking both RAR alpha and RAR gamma, as well as in RAR beta-null mice. All RAR-mediated signalling pathways are therefore dispensable in epidermis for homeostatic keratinocyte renewal. However, topical treatment of mouse skin with selective retinoids indicated that RXR/RAR gamma heterodimers, in which RXR transcriptional activity was subordinated to that of its RAR gamma partner, were required for retinoid-induced epidermal hyperplasia, whereas RXR homodimers and RXR/RAR alpha heterodimers were not involved. RA-induced keratinocyte proliferation was studied in mutant mice in which RXR alpha, RXR alpha and RAR alpha, RAR gamma, or RXR alpha and RAR gamma genes were specifically disrupted in either basal or suprabasal keratinocytes. We demonstrate that the topical retinoid signal is transduced by RXR alpha/RAR gamma heterodimers in suprabasal keratinocytes, which, in turn, stimulate proliferation of basal keratinocytes via a paracrine signal that may be heparin-binding EGF-like growth factor.
Figures



References
-
- Billoni N., Buan,B., Gautier,B., Gaillard,O., Mahé,Y.F. and Bernard,B.A. (2000) Thyroid hormone receptor β1 is expressed in the human hair follicle. Br. J. Dermatol., 142, 645–652. - PubMed
-
- Chambon P. (1996) A decade of molecular biology of retinoic acid receptors. FASEB J., 10, 940–954. - PubMed
-
- Chapellier B., Mark,M., Garnier,J.M., LeMeur,M., Chambon,P. and Ghyselinck,N.B. (2002a) A conditional floxed (loxP-flanked) allele for the Retinoic Acid Receptor alpha (RARα) gene. Genesis, 32, 87–89. - PubMed
-
- Chapellier B., Mark,M., Garnier,J.M., Dierich,A., Chambon,P. and Ghyselinck,N.B. (2002b) A conditional floxed (loxP-flanked) allele for the Retinoic Acid Receptor gamma (RARγ) gene. Genesis, 32, 95–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

