Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase
- PMID: 12093878
- PMCID: PMC2194017
- DOI: 10.1084/jem.20012094
Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase
Abstract
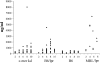
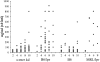
Mice lacking the membrane tyrosine kinase c-mer have been shown to have altered macro-phage cytokine production and defective phagocytosis of apoptotic cells despite normal phagocytosis of other particles. We show here that c-mer-deficient mice have impaired clearance of infused apoptotic cells and that they develop progressive lupus-like autoimmunity, with antibodies to chromatin, DNA, and IgG. The autoimmunity appears to be driven by endogenous antigens, with little polyclonal B cell activation. These mice should be an excellent model for studying the role of apoptotic debris as an immunogenic stimulus for systemic autoimmunity.
Figures



References
-
- Fadok, V.A., D.L. Bratton, D.M. Rose, A. Pearson, R.A. Ezekewitz, and P.M. Henson. 2000. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 405:85–90. - PubMed
-
- Surh, C.D., and J. Sprent. 1994. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 372:100–103. - PubMed
-
- Botto, M., C. Dell'Agnola, A.E. Bygrave, E.M. Thompson, H.T. Cook, F. Petry, M. Loos, P.P. Pandolfi, and M.J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56–59. - PubMed
-
- Camenisch, T.D., B.H. Koller, H.S. Earp, and G.K. Matsushima. 1999. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 162:3498–3503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

