G(1) and G(2) cell-cycle arrest following microtubule depolymerization in human breast cancer cells
- PMID: 12093892
- PMCID: PMC151025
- DOI: 10.1172/JCI13275
G(1) and G(2) cell-cycle arrest following microtubule depolymerization in human breast cancer cells
Abstract
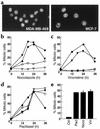
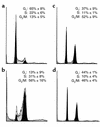
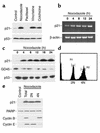
Microtubule-depolymerizing agents are widely used to synchronize cells, screen for mitotic checkpoint defects, and treat cancer. The present study evaluated the effects of these agents on normal and malignant human breast cell lines. After treatment with 1 microM nocodazole, seven of ten breast cancer lines (type A cells) arrested in mitosis, whereas the other three (type B cells) did not. Similar effects were observed with 100 nM vincristine or colchicine. Among five normal mammary epithelial isolates, four exhibited type A behavior and one exhibited type B behavior. Further experiments revealed that the type B cells exhibited a biphasic dose-response curve, with mitotic arrest at low drug concentrations (100 nM nocodazole or 6 nM vincristine) that failed to depolymerize microtubules and a p53-independent p21(waf1/cip1)-associated G(1) and G(2) arrest at higher concentrations (1 microM nocodazole or 100 nM vincristine) that depolymerized microtubules. Collectively, these observations provide evidence for coupling of premitotic cell-cycle progression to microtubule integrity in some breast cancer cell lines (representing a possible "microtubule integrity checkpoint") and suggest a potential explanation for the recently reported failure of some cancer cell lines to undergo nocodazole-induced mitotic arrest despite intact mitotic checkpoint proteins.
Figures

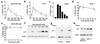
References
-
- Jordan MA, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol. 1998;10:123–130. - PubMed
-
- Downing KH. Structural basis for the interaction of tubulin with proteins and drugs that affect microtubule dynamics. Ann Rev Cell Dev Biol. 2000;16:89–111. - PubMed
-
- Rowinsky, E.K., and Donehower, R.C. 2001. Antimicrotubule agents. In Cancer chemotherapy and biotherapy. B.A. Chabner and D.L. Longo, editors. Lippincott, Williams & Wilkins. Philadelphia, Pennsylvania, USA. 329–372.
-
- Zieve GW, Turnbull D, Mullins JM, McIntosh JR. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Exp Cell Res. 1980;126:397–405. - PubMed
-
- De Brabander M, Geuens G, Nuydens R, Willebrords R, De Mey J. Microtubule assembly in living cells after release from nocodazole block: the effects of metabolic inhibitors, taxol and pH. Cell Biol Int Rep. 1981;5:913–920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

