Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP
- PMID: 12093909
- PMCID: PMC123176
- DOI: 10.1073/pnas.142213199
Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP
Abstract
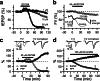
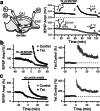
Hyperpolarization-activated nonselective cation channels (Ih channels) play an important role in the control of membrane excitability and rhythmic neuronal activity. The functional relevance of presynaptic Ih channels in regulating synaptic function, however, is not well established. Recently, it has been proposed [Mellor, J., Nicoll, R. A. & Schmitz, D. (2002) Science 295, 143-147] that presynaptic Ih channels are necessary for hippocampal mossy fiber long-term potentiation (LTP). This observation challenges an alternative model that suggests presynaptic forms of LTP are caused by a direct modification of the transmitter release machinery. Here, we assess the role of Ih in hippocampal mossy fiber LTP as well as cerebellar parallel fiber LTP, forms of potentiation that share common mechanisms. Our results show that after Ih blockade neither mossy fiber LTP nor parallel fiber LTP are affected. Furthermore, Ih does not significantly modify basal excitatory synaptic transmission in the hippocampus, whereas the organic Ih blockers ZD7288 and DK-AH 269 induce a large Ih-independent depression of synaptic transmission. In summary, our results indicate that Ih-mediated persistent changes in presynaptic excitability do not underlie presynaptic forms of LTP.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

