Promoter-proximal tethering elements regulate enhancer-promoter specificity in the Drosophila Antennapedia complex
- PMID: 12093913
- PMCID: PMC123125
- DOI: 10.1073/pnas.142291299
Promoter-proximal tethering elements regulate enhancer-promoter specificity in the Drosophila Antennapedia complex
Abstract
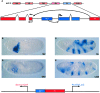
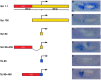
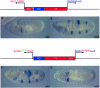
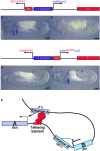
Insulator DNAs and promoter competition regulate enhancer-promoter interactions within complex genetic loci. Here we provide evidence for a third mechanism: promoter-proximal tethering elements. The Scr-ftz region of the Antennapedia gene complex includes two known enhancers, AE1 and T1. AE1 selectively interacts with the ftz promoter to maintain pair-rule stripes of ftz expression during gastrulation and germ-band elongation. The T1 enhancer, located 3' of the ftz gene and approximately 25 kb 5' of the Scr promoter, selectively activates Scr expression in the prothorax and posterior head segments. A variety of P element minigenes were examined in transgenic embryos to determine the basis for specific AE1-ftz and T1-Scr interactions. A 450-bp DNA fragment located approximately 100 bp 5' of the Scr transcription start site is essential for T1-Scr interactions and can mediate long-range activation of a ftz/lacZ reporter gene when placed 5' of the ftz promoter. We suggest that the Scr450 fragment contains tethering elements that selectively recruit T1 to the Scr promoter. Tethering elements might regulate enhancer-promoter interactions at other complex genetic loci.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

