The protofilament structure of insulin amyloid fibrils
- PMID: 12093917
- PMCID: PMC123117
- DOI: 10.1073/pnas.142459399
The protofilament structure of insulin amyloid fibrils
Abstract
Under solution conditions where the native state is destabilized, the largely helical polypeptide hormone insulin readily aggregates to form amyloid fibrils with a characteristic cross-beta structure. However, there is a lack of information relating the 4.8 A beta-strand repeat to the higher order assembly of amyloid fibrils. We have used cryo-electron microscopy (EM), combining single particle analysis and helical reconstruction, to characterize these fibrils and to study the three-dimensional (3D) arrangement of their component protofilaments. Low-resolution 3D structures of fibrils containing 2, 4, and 6 protofilaments reveal a characteristic, compact shape of the insulin protofilament. Considerations of protofilament packing indicate that the cross-beta ribbon is composed of relatively flat beta-sheets rather than being the highly twisted, beta-coil structure previously suggested by analysis of globular protein folds. Comparison of the various fibril structures suggests that very small, local changes in beta-sheet twist are important in establishing the long-range coiling of the protofilaments into fibrils of diverse morphology.
Figures



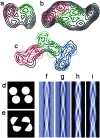
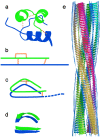
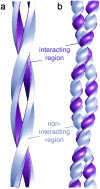
References
-
- Pepys M B. In: The Oxford Textbook of Medicine. 3rd Ed. Weatherall D J, Ledingham J G G, Warell D A, editors. Vol. 2. Oxford: Oxford Univ. Press; 1996. pp. 1512–1524.
-
- Sunde M, Serpell L C, Bartlam M, Fraser P E, Pepys M B, Blake C F. J Mol Biol. 1997;273:729–739. - PubMed
-
- Dobson C M. Trends Biochem Sci. 1999;24:329–332. - PubMed
-
- Cohen A S, Shirahama T, Skinner M. In: Electron Microscopy of Proteins. Harris J R, editor. Vol. 3. London: Academic; 1982. pp. 165–205.
-
- Serpell L C, Sunde M, Benson M D, Tennent G A, Pepys M B, Fraser P E. J Mol Biol. 2000;300:1033–1039. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

