Transport of volatile solutes through AQP1
- PMID: 12096045
- PMCID: PMC2290406
- DOI: 10.1113/jphysiol.2002.023218
Transport of volatile solutes through AQP1
Abstract
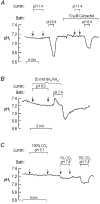
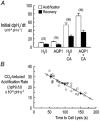
For almost a century it was generally assumed that the lipid phases of all biological membranes are freely permeable to gases. However, recent observations challenge this dogma. The apical membranes of epithelial cells exposed to hostile environments, such as gastric glands, have no demonstrable permeability to the gases CO2 and NH3. Additionally, the water channel protein aquaporin 1 (AQP1), expressed at high levels in erythrocytes, can increase membrane CO2 permeability when expressed in Xenopus oocytes. Similarly, nodulin-26, which is closely related to AQP1, can act as a conduit for NH3. A key question is whether aquaporins, which are abundant in virtually every tissue that transports O2 and CO2 at high levels, ever play a physiologically significant role in the transport of small volatile molecules. Preliminary data are consistent with the hypothesis that AQP1 enhances the reabsorption of HCO3- by the renal proximal tubule by increasing the CO2 permeability of the apical membrane. Other preliminary data on Xenopus oocytes heterologously expressing the electrogenic Na+-HCO3- cotransporter (NBC), AQP1 and carbonic anhydrases are consistent with the hypothesis that the macroscopic cotransport of Na+ plus two HCO3- occurs as NBC transports Na+ plus CO3(2-) and AQP1 transports CO2 and H2O. Although data - obtained on AQP1 reconstituted into liposomes or on materials from AQP1 knockout mice - appear inconsistent with the model that AQP1 mediates substantial CO2 transport in certain preparations, the existence of unstirred layers or perfusion-limited conditions may have masked the contribution of AQP1 to CO2 permeability.
Figures

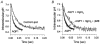
Comment in
-
Does aquaporin-1 pass gas? An opposing view.J Physiol. 2002 Jul 1;542(Pt 1):31. doi: 10.1113/jphysiol.2002.024398. J Physiol. 2002. PMID: 12096046 Free PMC article. No abstract available.
References
-
- Benga G, Pop VI, Popescu O, Ionescu M, Mihele V. Water exchange through erythrocyte membranes: nuclear magnetic resonance studies on the effects of inhibitors and of chemical modifications of human membranes. Journal of Membrane Biology. 1983;76:129–137. - PubMed
-
- Boron WF, Cooper GJ. Effect of DIDS on the CO2 permeability of the water channel AQP1. FASEB Journal. 1998;12:A374.
-
- Burckhardt BC, Frömter E. Pathways of NH3/NH4+ permeation across Xenopus laevis oocyte cell membrane. Pflügers Archiv. 1992;420:83–86. - PubMed
-
- Burg M, Grantham J, Abramow M, Orloff J. Preparation and study of fragments of single rabbit nephrons. American Journal of Physiology. 1966;210:1293–1298. - PubMed
-
- Cooper GJ, Boron WF. Effect of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. American Journal of Physiology. 1998a;275:C1481–1486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

