Water pumps
- PMID: 12096049
- PMCID: PMC2290409
- DOI: 10.1113/jphysiol.2002.018713
Water pumps
Abstract
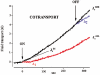
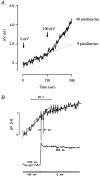
The transport of water across epithelia has remained an enigma ever since it was discovered over 100 years ago that water was transported across the isolated small intestine in the absence of osmotic and hydrostatic pressure gradients. While it is accepted that water transport is linked to solute transport, the actual mechanisms are not well understood. Current dogma holds that active ion transport sets up local osmotic gradients in the spaces between epithelial cells, the lateral intercellular spaces, and this in turn drives water transport by local osmosis. In the case of the small intestine, which in humans absorbs about 8 l of water a day, there is no direct evidence for either local osmosis or aquaporin gene expression in enterocytes. Intestinal water absorption is greatly enhanced by glucose, and this is the basis for oral rehydration therapy in patients with secretory diarrhoea. In our studies of the intestinal brush border Na+-glucose cotransporter we have obtained evidence that there is a direct link between the transport of Na+, glucose and water transport, i.e. there is cotransport of water along with Na+ and sugar, that will account for about 50 % of the total water transport across the human intestinal brush border membrane. In this short review we summarize the evidence for water cotransport and propose how this occurs during the enzymatic turnover of the transporter. This is a general property of cotransporters and so we expect that this may have wider implications in the transport of water and other small polar molecules across cell membranes in animals and plants.
Figures


Comment in
-
The presence of local osmotic gradients can account for the water flux driven by the Na+-glucose cotransporter.J Physiol. 2002 Jul 1;542(Pt 1):61-2. doi: 10.1113/jphysiol.2002.013328. J Physiol. 2002. PMID: 12096050 Free PMC article. No abstract available.
References
-
- Curran PF, McIntosh JR. A model system for biological water transport. Nature. 1962;193:347–348. - PubMed
-
- Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DDF. Inhibition of gap junction hemichannels by chloride channel blockers. Journal of Membrane Biology. 2002;185:93–102. - PubMed
-
- Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature. 1987;330:379–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

