Dual and opposing roles of presynaptic Ca2+ influx for spontaneous GABA release from rat medial preoptic nerve terminals
- PMID: 12096057
- PMCID: PMC2290392
- DOI: 10.1113/jphysiol.2001.015610
Dual and opposing roles of presynaptic Ca2+ influx for spontaneous GABA release from rat medial preoptic nerve terminals
Abstract
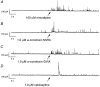
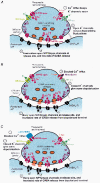
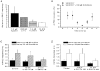
Calcium influx into the presynaptic nerve terminal is well established as a trigger signal for transmitter release by exocytosis. By studying dissociated preoptic neurons with functional adhering nerve terminals, we here show that presynaptic Ca2+ influx plays dual and opposing roles in the control of spontaneous transmitter release. Thus, application of various Ca2+ channel blockers paradoxically increased the frequency of spontaneous (miniature) inhibitory GABA-mediated postsynaptic currents (mIPSCs). Similar effects on mIPSC frequency were recorded upon washout of Cd2+ or EGTA from the external solution. The results are explained by a model with parallel Ca2+ influx through channels coupled to the exocytotic machinery and through channels coupled to Ca2+-activated K+ channels at a distance from the release site.
Figures


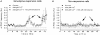
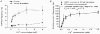

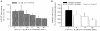
Similar articles
-
Heterogeneous presynaptic Ca2+ channel types triggering GABA release onto medial preoptic neurons from rat.J Physiol. 1998 Feb 15;507 ( Pt 1)(Pt 1):77-91. doi: 10.1111/j.1469-7793.1998.077bu.x. J Physiol. 1998. PMID: 9490820 Free PMC article.
-
Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: in vitro slice and synaptosome studies.Neuroscience. 2004;129(3):703-18. doi: 10.1016/j.neuroscience.2004.08.020. Neuroscience. 2004. PMID: 15541891
-
Effects of various K+ channel blockers on spontaneous glycine release at rat spinal neurons.Brain Res. 2007 Jul 9;1157:11-22. doi: 10.1016/j.brainres.2006.09.097. Epub 2007 Jun 6. Brain Res. 2007. PMID: 17555723
-
Presynaptic calcium channels: pharmacology and regulation.Neurochem Int. 1995 Jun;26(6):539-58. doi: 10.1016/0197-0186(94)00149-o. Neurochem Int. 1995. PMID: 7670358 Review.
-
Calcium channels at the photoreceptor synapse.Adv Exp Med Biol. 2002;514:465-76. doi: 10.1007/978-1-4615-0121-3_28. Adv Exp Med Biol. 2002. PMID: 12596939 Review.
Cited by
-
Nifedipine facilitates neurotransmitter release independently of calcium channels.Proc Natl Acad Sci U S A. 2003 May 13;100(10):6139-44. doi: 10.1073/pnas.0936131100. Epub 2003 Apr 24. Proc Natl Acad Sci U S A. 2003. PMID: 12714685 Free PMC article.
-
Presynaptic glycine receptors on GABAergic terminals facilitate discharge of dopaminergic neurons in ventral tegmental area.J Neurosci. 2004 Oct 13;24(41):8961-74. doi: 10.1523/JNEUROSCI.2016-04.2004. J Neurosci. 2004. PMID: 15483115 Free PMC article.
-
Simultaneous Release of Multiple Vesicles from Rods Involves Synaptic Ribbons and Syntaxin 3B.Biophys J. 2020 Feb 25;118(4):967-979. doi: 10.1016/j.bpj.2019.10.006. Epub 2019 Oct 10. Biophys J. 2020. PMID: 31653448 Free PMC article.
-
Hypothalamic and dietary control of temperature-mediated longevity.Ageing Res Rev. 2010 Jan;9(1):41-50. doi: 10.1016/j.arr.2009.07.004. Epub 2009 Jul 23. Ageing Res Rev. 2010. PMID: 19631766 Free PMC article. Review.
References
-
- Behrens R, Nolting A, Reimann F, Schwarz M, Waldschütz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel β subunit family. FEBS Letters. 2000;474:99–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

