In vitro cultivation of microsporidia of clinical importance
- PMID: 12097248
- PMCID: PMC118077
- DOI: 10.1128/CMR.15.3.401-413.2002
In vitro cultivation of microsporidia of clinical importance
Abstract
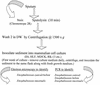
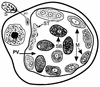
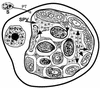
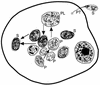
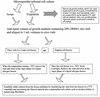
Although attempts to develop methods for the in vitro cultivation of microsporidia began as early as 1937, the interest in the culture of these organisms was confined mostly to microsporidia that infect insects. The successful cultivation in 1969 of Encephalitozoon cuniculi, a microsporidium of mammalian origin, and the subsequent identification of these organisms as agents of human disease heightened interest in the cultivation of microsporidia. I describe the methodology as well as the cell lines, the culture media, and culture conditions used in the in vitro culture of microsporidia such as Brachiola (Nosema) algerae, Encephalitozoon cuniculi, E. hellem, E. intestinalis, Enterocytozoon bieneusi, Trachipleistophora hominis, and Vittaforma corneae that cause human disease.
Figures





References
-
- Bocket, L., C. H. Marquette, A. Dewilde, D. Hober, and P. Wattre. 1992. Isolation and replication in human fibroblast cell (MRC-5) of a microsporidian from an AIDS patient. Microb. Pathog. 12:187-191. - PubMed
-
- Canning, E. U., and J. Lom. 1986. The microsporidia of vertebrates. Academic Press, Inc., New York, N.Y.
-
- Croppo, G. P., G. S. Visvesvara, G. J. Leitch, S. Wallace, and M. A. De Groote. 1997. Western blot and immunofluorescence analysis of a human isolate of Encephalitozoon cuniculi established in culture from the urine of a patient with AIDS. J. Parasitol. 83:66-69. - PubMed
-
- Croppo, G. P., G. S. Visvesvara, G. J. Leitch, S. Wallace, and D. A. Schwartz. 1998. Western blot identification of the microsporidian Encephalitozoon hellem using immunoglobulin G monoclonal antibodies. Arch. Pathol. Lab. Med. 122:182-186. - PubMed
-
- De Groote, M. A., G. S. Visvesvara, M. L. Wilson, N. J. Pieniazek, S. B. Slemenda, A. J. da Silva, G. J. Leitch, R. T. Bryan, and R. Reves. 1995. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J. Infect. Dis. 171:1375-1378. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

