In Vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis
- PMID: 12097494
- PMCID: PMC6758230
- DOI: 10.1523/JNEUROSCI.22-13-05423.2002
In Vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis
Abstract
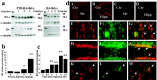
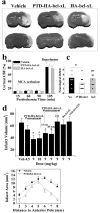
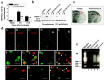
Bcl-xL is a well characterized death-suppressing molecule of the Bcl-2 family. Bcl-xL is expressed in embryonic and adult neurons of the CNS and may play a critical role in preventing neuronal apoptosis that occurs during brain development or results from diverse pathologic stimuli, including cerebral ischemia. In this study, we used a novel approach to study the potential neuroprotective effect of Bcl-xL as a therapeutic agent in the murine model of focal ischemia/reperfusion. We created a Bcl-xL fusion protein, designated as PTD-HA-Bcl-xL, which contains the protein transduction domain (PTD) derived from the human immunodeficiency TAT protein. We demonstrated that this fusion protein is highly efficient in transducing into primary neurons in cultures and potently inhibited staurosporin-induced neuronal apoptosis. Furthermore, intraperitoneal injection of PTD-HA-Bcl-xL into mice resulted in robust protein transduction in neurons in various brain regions within 1-2 hr, and decreased cerebral infarction (up to approximately 40%) in a dose-dependent manner, as determined at 3 d after 90 min of focal ischemia. PTD-HA-Bcl-xL was effective even when it was administered after the completion of ischemia (up to 45 min), and the protective effect was independent of the changes in cerebral blood flow or other physiological parameters. Finally, as shown by immunohistochemistry, Western blotting, and substrate-cleavage assays, PTD-HA-Bcl-xL attenuated ischemia-induced caspase-3 activation in ischemic neurons. These results thus confirm the neuroprotective effect of Bcl-xL against ischemic brain injury and provide the first evidence that the PTD can be used to efficiently transduce a biologically active neuroprotectant in experimental cerebral ischemia.
Figures

References
-
- Cao G, Minami M, Pei W, Yan C, Chen D, O'Horo C, Graham SH, Chen J. Intracellular Bax translocation after transient cerebral ischemia: implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. J Cereb Blood Flow Metab. 2001a;21:321–333. - PubMed
-
- Cao G, Luo Y, Nagayama T, Pei W, Stetler RA, Graham SH, Chen J. Cloning and characterization of rat caspase-9: implications for a role in mediating caspase-3 activation and hippocampal cell death after transient cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:534–546. - PubMed
-
- Chen D, Stetler RA, Cao G, Pei W, O'Horo C, Yin XM, Chen J. Characterization of the rat DNA fragmentation factor 35/inhibitor of caspase-activated DNase (short form): the endogenous inhibitor of caspase-dependent DNA fragmentation in neuronal apoptosis. J Biol Chem. 2000;275:38508–38517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
