A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons
- PMID: 12097496
- PMCID: PMC6758212
- DOI: 10.1523/JNEUROSCI.22-13-05442.2002
A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons
Abstract
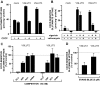
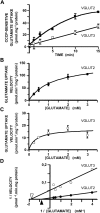
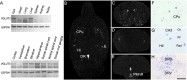
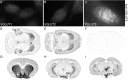
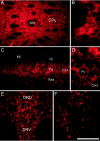
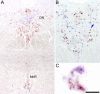
Two proteins previously known as Na(+)-dependent phosphate transporters have been identified recently as vesicular glutamate transporters (VGLUT1 and VGLUT2). Together, VGLUT1 and VGLUT2 are operating at most central glutamatergic synapses. In this study, we characterized a third vesicular glutamate transporter (VGLUT3), highly homologous to VGLUT1 and VGLUT2. Vesicles isolated from endocrine cells expressing recombinant VGLUT3 accumulated l-glutamate with bioenergetic and pharmacological characteristics similar, but not identical, to those displayed by the type-1 and type-2 isoforms. Interestingly, the distribution of VGLUT3 mRNA was restricted to a small number of neurons scattered in the striatum, hippocampus, cerebral cortex, and raphe nuclei, in contrast to VGLUT1 and VGLUT2 transcripts, which are massively expressed in cortical and deep structures of the brain, respectively. At the ultrastructural level, VGLUT3 immunoreactivity was concentrated over synaptic vesicle clusters present in nerve endings forming asymmetrical as well as symmetrical synapses. Finally, VGLUT3-positive neurons of the striatum and raphe nuclei were shown to coexpress acetylcholine and serotonin transporters, respectively. Our study reveals a novel class of glutamatergic nerve terminals and suggests that cholinergic striatal interneurons and serotoninergic neurons from the brainstem may store and release glutamate.
Figures



References
-
- Aihara Y, Mashima H, Onda H, Hisano S, Kasuya H, Hori T, Yamada S, Tomura H, Yamada Y, Inoue I, Kojima I, Takeda J. Molecular cloning of a novel brain-type Na+-dependent inorganic phosphate cotransporter. J Neurochem. 2000;74:2622–2625. - PubMed
-
- Antunes Bras JM, Epstein AL, Bourgoin S, Hamon M, Cesselin F, Pohl M. Herpes simplex virus 1-mediated transfer of preproenkephalin A in rat dorsal root ganglia. J Neurochem. 1998;70:1299–1303. - PubMed
-
- Bai L, Xu H, Collins JF, Ghishan FK. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J Biol Chem. 2001;276:36764–36769. - PubMed
-
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
