Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact
- PMID: 12097502
- PMCID: PMC6758202
- DOI: 10.1523/JNEUROSCI.22-13-05505.2002
Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact
Abstract
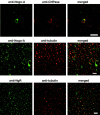
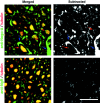
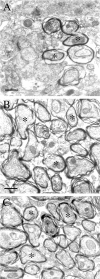
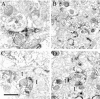
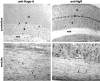
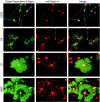
Axon regeneration in the adult CNS is limited by the presence of inhibitory proteins. An interaction of Nogo on the oligodendrocyte surface with Nogo-66 Receptor (NgR) on axons has been suggested to play an important role in limiting axonal growth. Here, we compare the localization of these two proteins immunohistochemically as a test of this hypothesis. Throughout much of the adult CNS, Nogo-A is detected on oligodendrocyte processes surrounding myelinated axons, including areas of axon-oligodendrocyte contact. The NgR protein is detected selectively in neurons and is present throughout axons, indicating that Nogo-A and its receptor are juxtaposed along the course of myelinated fibers. NgR protein expression is restricted to postnatal neurons and their axons. In contrast, Nogo-A is observed in myelinating oligodendrocytes, embryonic muscle, and neurons, suggesting that Nogo-A has additional physiologic roles unrelated to NgR binding. After spinal cord injury, Nogo-A is upregulated to a moderate degree, whereas NgR levels are maintained at constant levels. Taken together, these data confirm the apposition of Nogo ligand and NgR receptor in situations of limited axonal regeneration and support the hypothesis that this system regulates CNS axonal plasticity and recovery from injury.
Figures







References
-
- Benfey M, Aguayo AJ. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature. 1982;296:150–152. - PubMed
-
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. - PubMed
-
- Brittis PA, Flanagan JG. Nogo domains and a Nogo receptor: implications for axon regeneration. Neuron. 2001;30:11–14. - PubMed
-
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
