Targeted disruption of RC3 reveals a calmodulin-based mechanism for regulating metaplasticity in the hippocampus
- PMID: 12097504
- PMCID: PMC6758189
- DOI: 10.1523/JNEUROSCI.22-13-05525.2002
Targeted disruption of RC3 reveals a calmodulin-based mechanism for regulating metaplasticity in the hippocampus
Abstract
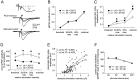
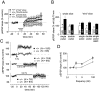
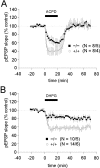
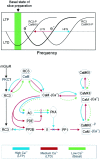
We used homologous recombination in the mouse to knock-out RC3, a postsynaptic, calmodulin-binding PKC substrate. Mutant brains exhibited lower immunoreactivity to phospho-Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) but had the same synaptic density as wild type and did not exhibit a gross neuroanatomical phenotype. Basal excitatory synaptic transmission in CA1 was depressed, long-term potentiation (LTP) was enhanced, and the depressant effects of the metabotropic glutamate receptor (mGluR) agonist (RS)-3,5-dihydroxyphenylglycine was occluded compared with littermate controls. The frequency-response curve was displaced to the left, and long-term depression (LTD) could not be induced unless low-frequency stimuli were preceded by high-frequency tetani. Depotentiation was much more robust in the mutant, and only one stimulus was required to saturate LTD in primed mutant hippocampi, whereas multiple low-frequency stimuli were required in wild-type slices. Thus, ablation of RC3 appears to render the postsynaptic neuron hypersensitive to Ca(2+), decreasing its LTD and LTP thresholds and accentuating the effects of priming stimuli. We propose an mGluR-dependent CaM-based sliding threshold mechanism for metaplasticity that is governed by the phosphorylation states of RC3 and CaMKII.
Figures




References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. - PubMed
-
- Artola A, Brocher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. - PubMed
-
- Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
