Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor
- PMID: 12097508
- PMCID: PMC6758233
- DOI: 10.1523/JNEUROSCI.22-13-05572.2002
Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor
Abstract
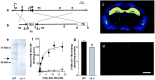
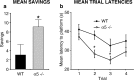
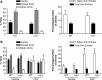
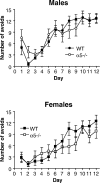
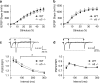
The alpha5 subunit of the GABA(A) receptor is localized mainly to the hippocampus of the mammalian brain. The significance of this rather distinct localization and the function of alpha5-containing GABA(A) receptors has been explored by targeted disruption of the alpha5 gene in mice. The alpha5 -/- mice showed a significantly improved performance in a water maze model of spatial learning, whereas the performance in non-hippocampal-dependent learning and in anxiety tasks were unaltered in comparison with wild-type controls. In the CA1 region of hippocampal brain slices from alpha5 -/- mice, the amplitude of the IPSCs was decreased, and paired-pulse facilitation of field EPSP (fEPSP) amplitudes was enhanced. These data suggest that alpha5-containing GABA(A) receptors play a key role in cognitive processes by controlling a component of synaptic transmission in the CA1 region of the hippocampus.
Figures


References
-
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKC gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. - PubMed
-
- Barnard EA, Skolnick P, Olson RW, Möhler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. - PubMed
-
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent K+ conductance. Nature. 2001;409:88–92. - PubMed
-
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy J-M, Luscher B, Möhler H. Decreased GABAA receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. - PubMed
-
- Culiat CT, Stubbs L, Woychik RP, Russell LB, Johnson DK, Rinchik EM. Deficiency of the β3 subunit of the type A γ-aminobutyric acid receptor causes cleft palate in mice. Nat Genet. 1995;11:344–346. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
