mu-Opioid receptors: Ligand-dependent activation of potassium conductance, desensitization, and internalization
- PMID: 12097530
- PMCID: PMC6758217
- DOI: 10.1523/JNEUROSCI.22-13-05769.2002
mu-Opioid receptors: Ligand-dependent activation of potassium conductance, desensitization, and internalization
Abstract
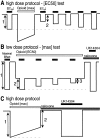
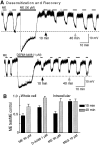
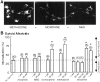
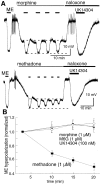
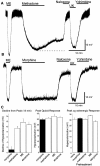
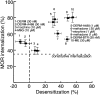
micro-Opioid receptor (MOR) desensitization and endocytosis have been implicated in tolerance and dependence to opioids. The efficiency of each process is known to be agonist dependent; however, it is not known what determines the relative efficiency of various agonists at either process. In the present study, homologous MOR desensitization in locus ceruleus (LC) neurons and MOR internalization in HEK293 cells were examined using a series of agonists. The results show that the rank order of this series of agonists was different when comparing the magnitude of hyperpolarization and the ability to cause desensitization in LC neurons. Endocytosis of MOR was also examined in HEK293 cells using the same agonists. The relative ability to cause endocytosis in HEK293 cells correlated with the degree of desensitization in LC cells. This strong correlation suggests that the two processes are closely linked. The results also suggest that agonist efficacy is not necessarily a predictor of the ability to cause MOR desensitization or endocytosis. Identification and characterization of the biophysical properties of agonists that favor desensitization and internalization of receptors will lead to a better understanding of opioid signaling.
Figures


References
-
- Arttamangkul S, Alvarez-Maubecin V, Thomas G, Williams JT, Grandy DK. Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol Pharmacol. 2000;58:1570–1580. - PubMed
-
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. - PubMed
-
- Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. - PubMed
-
- Gomes BA, Shen J, Stafford K, Patel M, Yoburn BC. μ-Opioid receptor down-regulation and tolerance are not equally dependent upon G-protein signaling. Pharmacol Biochem Behav. 2002;72:273–278. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
