Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users
- PMID: 12097556
- PMCID: PMC136380
- DOI: 10.1128/jvi.76.15.7444-7452.2002
Intersubtype human immunodeficiency virus type 1 superinfection following seroconversion to primary infection in two injection drug users
Abstract
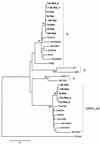
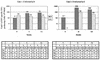
In this study, we describe two cases of human immunodeficiency virus type 1 (HIV-1) intersubtype superinfection with CRF01_AE and subtype B strains, which occurred in two injection drug users participating in a prospective cohort study in Bangkok, Thailand. In both cases, the superinfecting strain was detected by molecular and serologic analyses several weeks after complete seroconversion to the primary infection with a strain belonging to a different subtype. Superinfection occurred despite specific T-cell and humoral antibody responses to the primary virus. In both cases, cross-subtype immune responses were limited or absent prior to the second infection. These data show that, in some individuals, the quality and quantity of the immune response elicited by primary HIV-1 infection may not protect against superinfection. This finding has important implications for vaccine design. HIV-1 vaccines, at a minimum, will need to include potent, broadly protective, conserved immunogens derived from several group M subtypes.
Figures

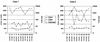

References
-
- Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 274:54-60. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Artenstein, A. W., T. C. VanCott, J. R. Mascola, J. K. Carr, P. A. Hegerich, J. Gaywee, E. Sanders-Buell, M. L. Robb, D. E. Dayhoff, S. Thitivichianlert, S. Nitayaphan, J. G. McNeil, D. L. Birx, R. A. Michael, D. S. Burke, and F. E. McCutchan. 1995. Dual infection with human immunodeficiency virus type 1 of distinct envelope subtypes in humans. J. Infect. Dis. 171:805-810. - PubMed
-
- Balla-Jhagjhoorsingh, S. S., P. Mooij, P. J. ten Haaft, W. M. Bogers, V. J. Teeuwsen, G. Koopman, and J. L. Heeney. 2001. Protection from secondary human immunodeficiency virus type 1 infection in chimpanzees suggests the importance of antigenic boosting and a possible role for cytotoxic T cells. J. Infect. Dis. 184:136-143. - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented vaccination. Science 290:486-492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

