Contribution of protein p40 to hypovirus-mediated modulation of fungal host phenotype and viral RNA accumulation
- PMID: 12097588
- PMCID: PMC136391
- DOI: 10.1128/jvi.76.15.7747-7759.2002
Contribution of protein p40 to hypovirus-mediated modulation of fungal host phenotype and viral RNA accumulation
Abstract
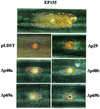
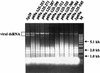
The papain-like protease p29, derived from the N-terminal portion of the hypovirus CHV1-EP713-encoded open reading frame (ORF) A polyprotein, p69, was previously shown to contribute to reduced pigmentation and sporulation by the infected host, the chestnut blight fungus Cryphonectria parasitica, while being dispensable for virus replication and attenuation of fungal virulence (hypovirulence). We now report that deletion of the C-terminal portion of p69, which encodes the highly basic protein p40, resulted in replication-competent mutant viruses that were, however, significantly reduced in RNA accumulation. While the Delta p40 mutants retained the ability to confer hypovirulence, Delta p40-infected fungal strains produced more asexual spores than strains infected with either wild-type CHV1-EP713 or a Delta p29 mutant virus. As observed for Delta p29-infected colonies, pigment production was significantly increased in Delta p40-infected fungal strains relative to that in CHV1-EP713-infected strains. Virus-mediated suppression of laccase production was not affected by p40 deletion. A gain-of-function analysis was employed to map the p40 symptom determinant to the N-terminal domain, encompassing p69 amino acid residues Thr(288) to Arg(312). Evidence that the gain of function was due to the encoded protein rather than the corresponding RNA sequence element was provided by introducing frameshift mutations on either side of the activity determinant domain. Moreover, restoration of symptoms correlated with increased accumulation of viral RNA. These results suggest that p40 indirectly contributes to virus-mediated suppression of fungal pigmentation and conidiation by providing an accessory function in hypovirus RNA amplification. A possible role for p40 in facilitating ORF B expression and the relationship between hypovirus RNA accumulation and symptom expression are discussed.
Figures





References
-
- Anagnostakis, S. L. 1982. Biological control of chestnut blight. Science 215:466-471. - PubMed
-
- Asamizu, T., D. Summers, M. B. Motika, J. V. Anzola, and D. L. Nuss. 1985. Molecular cloning and characterization of the genome of wound tumor virus: a tumor-inducing plant reovirus. Virology 144:398-409. - PubMed
-
- Baltimore, D. 1969. The replication of picornaviruses, p. 101-176. In H. B. Levy (ed.), The biochemistry of viruses. Marcel Dekker, New York, N.Y.
-
- Bavendamm, W. 1928. Uber das vorkommen und den Nachweis von Oxydasen bei holzzerstorenden Pilzen. Z. Pflanzenkr. Pflanzenschutz 38:257-276.
-
- Chen, B., G. H. Choi, and D. L. Nuss. 1994. Attenuation of fungal virulence by synthetic infectious hypovirus transcripts. Science 264:1762-1764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

