Similarities in the induction of post-Golgi vesicles by the vaccinia virus F13L protein and phospholipase D
- PMID: 12097590
- PMCID: PMC136368
- DOI: 10.1128/jvi.76.15.7777-7789.2002
Similarities in the induction of post-Golgi vesicles by the vaccinia virus F13L protein and phospholipase D
Abstract
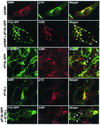
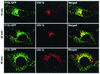
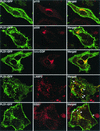
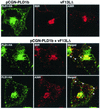
Intracellular mature vaccinia virions are wrapped by cisternae, derived from virus-modified trans-Golgi or endosomal membranes, and then transported via microtubules to the cell periphery. Two viral proteins, encoded by the F13L and B5R open reading frames, are essential for the membrane-wrapping step. Previous transfection studies indicated that F13L induces the formation of post-Golgi vesicles that incorporate the B5R protein and that this activity depends on an intact F13L phospholipase motif. Here we show that the F13L protein has a general effect on the trafficking of integral membrane proteins from the Golgi apparatus, as both the vaccinia virus A36R protein and the vesicular stomatitis virus G protein also colocalized with the F13L protein in vesicles. In addition, increased expression of cellular phospholipase D, which has a similar phospholipase motif as, but little amino acid sequence identity with, F13L, induced post-Golgi vesicles that contained B5R and A36R proteins. Butanol-1, which prevents the formation of phosphatidic acid by phospholipase D and specifically inhibits phospholipase D-mediated vesicle formation, also inhibited F13L-induced vesicle formation, whereas secondary and tertiary alcohols had no effect. Moreover, inhibition of phospholipase activity by butanol-1 also reduced plaque size and decreased the formation of extracellular vaccinia virus without affecting the yield of intracellular mature virus. Phospholipase D, however, could not complement a vaccinia virus F13L deletion mutant, indicating that F13L has additional virus-specific properties. Taken together, these data support an important role for F13L in inducing the formation of vesicle precursors of the vaccinia virus membrane via phospholipase activity or activation.
Figures




References
-
- Baek, S.-H., J.-Y. Kwak, S. H. Lee, T. Lee, S. H. Ryu, D. J. Uhlinger, and J. D. Lambeth. 1997. Lipase activities of p37, the major envelope protein of vaccinia virus. J. Biol. Chem. 272:32042-32049. - PubMed
-
- Bednarek, S. Y., L. Orici, and R. Schekman. 1996. Traffic COPS and the formation of vesicle coats. Trends Cell Biol. 6:468-473. - PubMed
-
- Brown, F. D., N. Thompson, K. M. Saqib, J. M. Clark, D. Powner, N. T. Thompson, R. Solari, and M. J. Wakelam. 1998. Phospholipase D1 localizes to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr. Biol. 8:835-838. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

