Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia
- PMID: 12097645
- PMCID: PMC126639
- DOI: 10.1073/pnas.152002699
Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia
Abstract
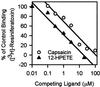
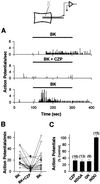
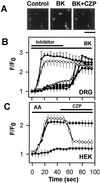
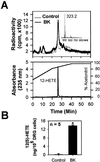
The capsaicin-sensitive vanilloid receptor (VR1) was recently shown to play an important role in inflammatory pain (hyperalgesia), but the underlying mechanism is unknown. We hypothesized that pain-producing inflammatory mediators activate capsaicin receptors by inducing the production of fatty acid agonists of VR1. This study demonstrates that bradykinin, acting at B2 bradykinin receptors, excites sensory nerve endings by activating capsaicin receptors via production of 12-lipoxygenase metabolites of arachidonic acid. This finding identifies a mechanism that might be targeted in the development of new therapeutic strategies for the treatment of inflammatory pain.
Figures



References
-
- Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature (London) 1997;389:816–824. - PubMed
-
- Tominaga M, Caterina M J, Malmberg A B, Rosen T A, Gilbert H, Skinner K, Raumann B E, Basbaum A I, Julius D. Neuron. 1998;21:531–543. - PubMed
-
- Caterina M J, Leffler A, Malmberg A B, Martin W J, Trafton J, Petersen-Zeitz K R, Koltzenburg M, Basbaum A I, Julius D. Science. 2000;288:306–313. - PubMed
-
- Davis J B, Gray J, Gunthorpe M J, Hatcher J P, Davey P T, Overend P, Harries M H, Latcham J, Clapham C, Atkinson K, et al. Nature (London) 2000;405:183–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

