Biotin uptake into human peripheral blood mononuclear cells increases early in the cell cycle, increasing carboxylase activities
- PMID: 12097659
- PMCID: PMC1435359
- DOI: 10.1093/jn/132.7.1854
Biotin uptake into human peripheral blood mononuclear cells increases early in the cell cycle, increasing carboxylase activities
Abstract
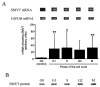
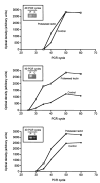
Cells respond to proliferation with increased accumulation of biotin, suggesting that proliferation enhances biotin demand. Here we determined whether peripheral blood mononuclear cells (PBMC) increase biotin uptake at specific phases of the cell cycle, and whether biotin is utilized to increase biotinylation of carboxylases. Biotin uptake was quantified in human PBMC that were arrested chemically at specific phases of the cell cycle, i.e., biotin uptake increased in the G1 phase of the cycle [658 +/- 574 amol biotin/(10(6) cells x 30 min)] and remained increased during phases S, G2, and M compared with quiescent controls [200 +/- 62 amol biotin/(10(6) cells x 30 min)]. The abundance of the sodium-dependent multivitamin transporter (SMVT, which transports biotin) was similar at all phases of the cell cycle, suggesting that transporters other than SMVT or splicing variants of SMVT may account for the increased biotin uptake observed in proliferating cells. Activities of biotin-dependent 3-methylcrotonyl-CoA carboxylase and propionyl-CoA carboxylase were up to two times greater in proliferating PBMC compared with controls. The abundance of mRNA encoding 3-methylcrotonyl-CoA carboxylase and propionyl-CoA carboxylase paralleled carboxylase activities, suggesting that PBMC respond to proliferation with increased expression of genes encoding carboxylases. Similarly, expression of the gene encoding holocarboxylase synthetase (which catalyzes binding of biotin to carboxylases) increased in response to proliferation, suggesting that cellular capacity to biotinylate carboxylases was increased. In summary, these findings suggest that PBMC respond to proliferation with increased biotin uptake early in the cell cycle, and that biotin is utilized to increase activities of two of the four biotin-requiring carboxylases.
Figures




References
-
- Zempleni J, Mock DM. Biotin biochemistry and human requirements. J Nutr Biochem. 1999;10:128 –138. - PubMed
-
- Wang H, Huang W, Fei YJ, Xia H, Fang-Yeng TL, Leibach FH, Devoe LD, Ganapathy V, Prasad PD. Human placental Na+-dependent multivitamin transporter. J Biol Chem. 1999;274:14875–14883. - PubMed
-
- Zempleni J, Mock DM. Uptake and metabolism of biotin by human peripheral blood mononuclear cells. Am J Physiol. 1998;275:C382–C388. - PubMed
-
- Zempleni J, Mock DM. Human peripheral blood mononuclear cells: inhibition of biotin transport by reversible competition with pantothenic acid is quantitatively minor. J Nutr Biochem. 1999;10:427–432. - PubMed
-
- Zempleni J, Mock DM. Mitogen-induced proliferation increases biotin uptake into human peripheral blood mononuclear cells. Am J Physiol. 1999;276:C1079 –C1084. - PubMed
