Barriers to sexual reproduction in Polygonum viviparum: a comparative developmental analysis of P. viviparum and P. bistortoides
- PMID: 12099345
- PMCID: PMC4233787
- DOI: 10.1093/aob/mcf020
Barriers to sexual reproduction in Polygonum viviparum: a comparative developmental analysis of P. viviparum and P. bistortoides
Abstract
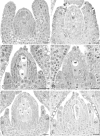
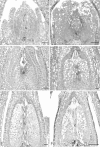
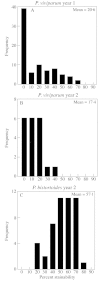
Polygonum viviparum is widely distributed in arctic and alpine regions of the northern hemisphere. Fruit set has never been observed in North American populations and has been reported only very rarely in Europe. Although this species is extremely well studied, the impediments to successful fruit production are unknown. We investigated the sexual reproductive process in P. viviparum growing in the southern Colorado Rocky Mountains. For comparison, we also examined this process in the sympatric congener P. bistortoides, in which reproduction is exclusively sexual. Lack of viable fruit production in P. viviparum has no single developmental explanation; defects occur in each of the processes and structures associated with sexual reproduction studied, yet, these processes and structures also appear to function normally in at least some flowers or individuals. Development is abnormal in many ovules of P. viviparum, however, comparison with P. bistortoides shows that these abnormalities do not contribute to differences in seed production between the two species. The virtual absence of sexual reproduction in P. viviparum appears to be due largely to a low rate of fertilization and to embryo/fruit abortion.
Figures


References
-
- AkhalkatsiM, Pfauth M, Calvin CL.1999. Structural aspects of ovule and seed abortion in Melilotus officinalis (Fabaceae). Protoplasma 208: 211–223.
-
- AlbertS, Despres B, Gilleminot J, Bechtold N, Pelletier G, Delseny M, Devic M.1999. The EMB 506 gene encodes a novel ankyrin repeat containing protein that is essential for the normal development of Arabidopsis embryos. Plant Journal 17: 169–179. - PubMed
-
- AnderssonS.1993. The potential for selective seed maturation in Achillea ptarmica (Asteraceae). Oikos 66: 36–42.
-
- BauertMR.1993. Vivipary in Polygonum viviparum: an adaptation to cold climate? Nordic Journal of Botany 13: 473–480.

