Hippocampal spatial representations require vestibular input
- PMID: 12099481
- PMCID: PMC1823522
- DOI: 10.1002/hipo.1112
Hippocampal spatial representations require vestibular input
Abstract
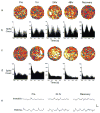
The hippocampal formation is essential for forming declarative representations of the relationships among multiple stimuli. The rodent hippocampal formation, including the entorhinal cortex and subicular complex, is critical for spatial memory. Two classes of hippocampal neurons fire in relation to spatial features. Place cells collectively map spatial locations, with each cell firing only when the animal occupies that cell's "place field," a particular subregion of the larger environment. Head direction (HD) cells encode directional heading, with each HD cell firing when the rat's head is oriented in that cell's particular "preferred firing direction." Both landmarks and internal cues (e.g., vestibular, motor efference copy) influence place and HD cell activity. However, as is the case for navigation, landmarks are believed to exert greater influence over place and HD cell activity. Here we show that temporary inactivation of the vestibular system led to the disruption of location-specific firing in hippocampal place cells and direction-specific discharge of postsubicular HD cells, without altering motor function. Place and HD cell activity recovered over a time course similar to that of the restoration of vestibular function. These results indicate that vestibular signals provide an important influence over the expression of hippocampal spatial representations, and may explain the navigational deficits of humans with vestibular dysfunction.
Figures

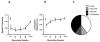
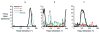
References
-
- Archey WB, Stackman RW, Goodridge JP, Dudchenko PA, Taube JS. Place cells show directionality in an open field following lesions of the head direction cell system. Soc Neurosci Abstr. 1997;23:504.
-
- Ballesteros MA, Gallo M. Bilateral tetrodotoxin blockade of the rat vestibular nuclei substitutes the natural unconditioned stimulus in taste aversion learning. Neurosci Lett. 2000;279:161–164. - PubMed
-
- Beitz AJ, Saxon DW, Anderson JH. Development of a novel, reversible labyrinthectomy model: behavioral and anatomical correlates. Soc Neurosci Abstr. 1995;21:919.
-
- Beritoff JS. Neural mechanisms of higher vertebrate behavior. Boston: Little, Brown and Co; 1966.
-
- Blair HT, Sharp PE. Visual and vestibular influences on head-direction cells in the anterior thalamus of the rat. Behav Neurosci. 1996;110:643– 660. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

