Deficiencies in CD4+ and CD8+ T cell subsets in ataxia telangiectasia
- PMID: 12100032
- PMCID: PMC1906431
- DOI: 10.1046/j.1365-2249.2002.01830.x
Deficiencies in CD4+ and CD8+ T cell subsets in ataxia telangiectasia
Abstract
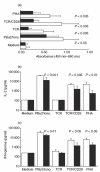
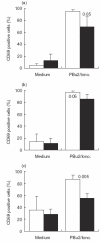
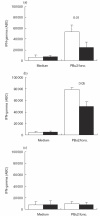
Chronic sinopulmonary infections that are associated with immunodeficiency are one of the leading causes of death in the multi-systemic disease ataxia telangiectasia (AT). Immunological investigations of AT patients revealed a broad spectrum of defects in the humoral and the cellular immune system. Based on their important role in host defence the aim of our study was an extensive analysis of cell distribution and function of CD4+ and CD8+ T lymphocytes and NK cells. We found that naive (CD45RA+) CD4+ lymphocytes, as well as CD8+/CD45RA+ lymphocytes, are decreased, whereas NK cells (CD3-/CD16+CD56+) are significantly elevated in AT patients. In our culture system proliferation and cytokine production was normal in purified memory (CD45RO+) lymphocytes after stimulation with phorbol-12,13-dibutyrate (PBu2) and after PHA activation, indicating that differences in proliferation and cytokine production are due solely to reduced numbers of CD45RA+ lymphocytes. However, activation, and especially intracellular interferon production of AT lymphocytes, seem to follow different kinetics compared to controls. In contrast to polyclonal activation, stimulation via the T cell receptor results consistently in a reduced immune response. Taken together, our results suggest that deficiency of immunocompetent cells and an intrinsic immune activation defect are responsible for the immunodeficiency in AT.
Figures



References
-
- Gatti RA, Boder E, Vinters HV, Sparkes RS, Norman A, Lange K. Ataxia-telangiectasia: an interdisciplinary approach to pathogenesis. Medicine. 1991;70:99–117. - PubMed
-
- Lavin F, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 1997;15:177–202. - PubMed
-
- Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia-telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–53. - PubMed
-
- Meyn MS. Ataxia-telangiectasia and cellular responses to DNA damage. Cancer Res. 1995;55:5991–6001. - PubMed
-
- Lavin F, Shiloh Y. Ataxia-telangiectasia: a multifaceted genetic disorder associated with defective signal transduction. Curr Opin Immunol. 1996;8:459–64. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

