Lupus-specific kidney deposits of HSP90 are associated with altered IgG idiotypic interactions of anti-HSP90 autoantibodies
- PMID: 12100037
- PMCID: PMC1906416
- DOI: 10.1046/j.1365-2249.2002.01887.x
Lupus-specific kidney deposits of HSP90 are associated with altered IgG idiotypic interactions of anti-HSP90 autoantibodies
Abstract
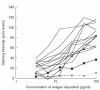
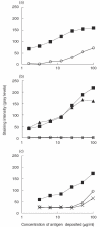
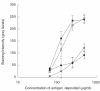
Previous studies have shown that autoantibodies to heat shock protein 90 (HSP90) are elevated in a significant proportion of patients with systemic lupus erythematosus (SLE) who are more likely to have renal disease and a low C3 level. Using samples from 24 patients, we searched for glomerular deposits of HSP90 in renal biopsy specimens from seven patients with lupus nephritis and 17 cases of glomerulonephritis from patients without SLE. Positive glomerular immunofluorescent staining for HSP90 was observed in six of seven cases of SLE and positive tubular staining in two of seven SLE patients. The staining for HSP90 was granular in nature and was located in subepithelial, subendothelial and mesangial areas. None of the non-SLE renal biopsies revealed positive staining for HSP90 deposition. Further we showed the presence of anti-HSP90 IgG autoantibodies in IgG from sera of patients with SLE as well as in normal human IgG (IVIg). In normal IgG this autoreactivity could be adsorbed almost completely on F(ab')2 fragments from the same IgG preparation, coupled to Sepharose and could be inhibited by the effluent obtained after subjecting normal IgG to HSP90 affinity column. These findings indicate that anti-HSP90 natural autoantibodies are blocked by idiotypic interactions within the IgG repertoire. Unlike natural autoantibodies, anti-HSP90 IgG from SLE patients' sera were only moderately adsorbed on F(ab')2 fragments of normal IgG. These results demonstrate that immunopathogenesis of lupus nephritis is associated with HSP90 (as an autoantigen) and that the pathology is associated with altered idiotypic regulation of the anti-HSP90 IgG autoantibodies.
Figures




References
-
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–91. - PubMed
-
- Conroy SE, Faulds GB, Williams W, Latchman DS, Isenberg DA. Detection of autoantibodies to the 90 kDa heat shock protein in systemic lupus erythematosus and other autoimmune diseases. Br J Rheumatol. 1994;33:923–6. - PubMed
-
- Erkeller-Yuksel FM, Isenberg DA, Dhillon VB, Latchman DS, Lydyard PM. Surface expression of heat shock protein 90 by blood mononuclear cells from patients with systemic lupus erythematosus. J Autoimmun. 1992;5:803–14. - PubMed
-
- Twomey BM, Dhillon VB, McCallum S, Isenberg DA, Latchman DS. Elevated levels of the 90 kD heat shock protein in patients with systemic lupus erythematosus are dependent upon enhanced transcription of the hsp90 beta gene. J Autoimmun. 1993;6:495–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

