Measurement of renal function during drug development
- PMID: 12100232
- PMCID: PMC1874392
- DOI: 10.1046/j.1365-2125.2002.01625.x
Measurement of renal function during drug development
Figures

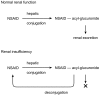

References
-
- Zaltzman JS, Whiteside C, Cattran DC, Lopez FM, Logan AG. Accurate measurement of impaired glomerular filtration using single-dose oral cimetidine. Am J Kidney Dis. 1996;27:504–511. - PubMed
-
- Roubenoff R, Drew H, Moyer M, Petri M, Whiting-O'Keefe Q, Hellmann DB. Oral cimetidine improves the accuracy and precision of creatinine clearance in lupus nephritis. Ann Intern Med. 1990;113:501–506. - PubMed
-
- Bianchi C, Donadio C, Tramonti G. Noninvasive methods for the measurement of total renal function. Nephron. 1981;28:53–57. - PubMed
-
- Levey AS. Measurement of renal function in chronic renal disease. Kidney Int. 1990;38:167–184. - PubMed
-
- Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

