Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon
- PMID: 12101127
- PMCID: PMC186370
- DOI: 10.1101/gad.231702
Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon
Abstract
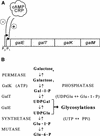
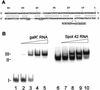
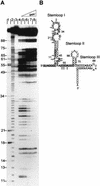
The physiological role of Escherichia coli Spot 42 RNA has remained obscure, even though the 109-nucleotide RNA was discovered almost three decades ago. Structural features of Spot 42 RNA and previous work suggested to us that the RNA might be a regulator of discoordinate gene expression of the galactose operon, a control that is only understood at the phenomenological level. The effects of controlled expression of Spot 42 RNA or deleting the gene (spf) encoding the RNA supported this hypothesis. Down-regulation of galK expression, the third gene in the gal operon, was only observed in the presence of Spot 42 RNA and required growth conditions that caused derepression of the spf gene. Subsequent biochemical studies showed that Spot 42 RNA specifically bound at the galK Shine-Dalgarno region of the galETKM mRNA, thereby blocking ribosome binding. We conclude that Spot 42 RNA is an antisense RNA that acts to differentially regulate genes that are expressed from the same transcription unit. Our results reveal an interesting mechanism by which the expression of a promoter distal gene in an operon can be modulated and underline the importance of antisense control in bacterial gene regulation.
Figures



References
-
- Adhya S. The galactose operon. In: In: Neidhardt FC, et al., editors. Escherichia coli and Salmonella thyphimurium: Cellular and molecular biology. Washington, DC.: American Society for Microbiology; 1987. pp. 1503–1512.
-
- Adoutte A. Evolutionary biology. Small but mighty timekeepers. Nature. 2000;408:37–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
