Jun dimerization protein 2 functions as a progesterone receptor N-terminal domain coactivator
- PMID: 12101239
- PMCID: PMC133955
- DOI: 10.1128/MCB.22.15.5451-5466.2002
Jun dimerization protein 2 functions as a progesterone receptor N-terminal domain coactivator
Abstract
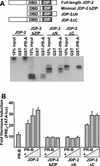
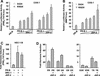
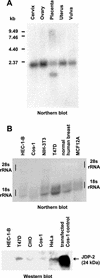
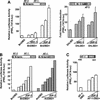
The progesterone receptor (PR) contains two transcription activation function (AF) domains, constitutive AF-1 in the N terminus and AF-2 in the C terminus. AF-2 activity is mediated by a hormone-dependent interaction with a family of steroid receptor coactivators (SRCs). SRC-1 can also stimulate AF-1 activity through a secondary domain that interacts simultaneously with the primary AF-2 interaction site. Other protein interactions and mechanisms that mediate AF-1 activity are not well defined. By interaction cloning, we identified an AP-1 family member, Jun dimerization protein 2 (JDP-2), as a novel PR-interacting protein. JDP-2 was first defined as a c-Jun interacting protein that functions as an AP-1 repressor. PR and JDP-2 interact directly in vitro through the DNA binding domain (DBD) of PR and the basic leucine zipper (bZIP) region of JDP-2. The two proteins also physically associate in mammalian cells, as detected by coimmunoprecipitation, and are recruited in vivo to a progesterone-inducible target gene promoter, as detected by a chromatin immunoprecipitation (ChIP) assay. In cell transfection assays, JDP-2 substantially increased hormone-dependent PR-mediated transactivation and worked primarily by stimulating AF-1 activity. JDP-2 is a substantially stronger coactivator of AF-1 than SRC-1 and stimulates AF-1 independent of SRC-1 pathways. The PR DBD is necessary but not sufficient for JDP-2 stimulation of PR activity; the DBD and AF-1 are required together. JDP-2 lacks an intrinsic activation domain and makes direct protein interactions with other coactivators, including CBP and p300 CBP-associated factor (pCAF), but not with SRCs. These results indicate that JDP-2 stimulates AF-1 activity by the novel mechanism of docking to the DBD and recruiting or stabilizing N-terminal PR interactions with other general coactivators. JDP-2 has preferential activity on PR among the nuclear receptors tested and is expressed in progesterone target cells and tissues, suggesting that it has a physiological role in PR function.
Figures




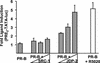
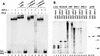
References
-
- Allan, G., S. Leng, S. Tsai, N. Weigel, D. Edwards, M.-J. Tsai, and B. O'Malley. 1992. Hormone and antihormone induce distinct conformational changes which are central to steroid receptor activation. J. Biol. Chem. 267:19513-19520. - PubMed
-
- Bain, D., M. Franden, J. McManaman, G. Takimoto, and K. Horwitz. 2000. The N-terminal region of the human progesterone A-receptor. J. Biol. Chem. 275:7313-7320. - PubMed
-
- Baudino, T., D. Kraichely, S. Jefcoat, Jr., S. Winchester, N. Partridge, and P. MacDonald. 1998. Isolation and characterization of a novel coactivator protein, NCoA-62, involved in vitamin D-mediated transcription. J. Biol. Chem. 273:16434-16441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
