Hematopoietic stem cell expansion and distinct myeloid developmental abnormalities in a murine model of the AML1-ETO translocation
- PMID: 12101243
- PMCID: PMC133929
- DOI: 10.1128/MCB.22.15.5506-5517.2002
Hematopoietic stem cell expansion and distinct myeloid developmental abnormalities in a murine model of the AML1-ETO translocation
Abstract
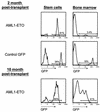
The t(8;21)(q22;q22) translocation, which fuses the ETO gene on human chromosome 8 with the AML1 gene on chromosome 21 (AML1-ETO), is one of the most frequent cytogenetic abnormalities associated with acute myelogenous leukemia (AML). It is seen in approximately 12 to 15% of AML cases and is present in about 40% of AML cases with a French-American-British classified M2 phenotype. We have generated a murine model of the t(8;21) translocation by retroviral expression of AML1-ETO in purified hematopoietic stem cells (HSC). Animals reconstituted with AML1-ETO-expressing cells recapitulate the hematopoietic developmental abnormalities seen in the bone marrow of human patients with the t(8;21) translocation. Primitive myeloblasts were increased to approximately 10% of bone marrow by 10 months posttransplant. Consistent with this observation was a 50-fold increase in myeloid colony-forming cells in vitro. Accumulation of late-stage metamyelocytes was also observed in bone marrow along with an increase in immature eosinophilic myelocytes that showed abnormal basophilic granulation. HSC numbers in the bone marrow of 10-month-posttransplant animals were 29-fold greater than in transplant-matched control mice, suggesting that AML1-ETO expression overrides the normal genetic control of HSC pool size. In summary, AMLI-ETO-expressing animals recapitulate many (and perhaps all) of the developmental abnormalities seen in human patients with the t(8;21) translocation, although the animals do not develop leukemia or disseminated disease in peripheral tissues like the liver or spleen. This suggests that the principal contribution of AML1-ETO to acute myeloid leukemia is the inhibition of multiple developmental pathways.
Figures
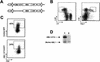
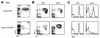




References
-
- Andrieu, V., I. Radford-Weiss, X. Troussard, C. Chane, F. Valensi, M. Guesnu, E. Haddad, F. Viguier, F. Dreyfus, B. Varet, G. Flandrin, and E. Macintyre. 1996. Molecular detection of t(8;21)/AML1-ETO in AML M1/M2: correlation with cytogenetics, morphology and immunophenotype. Br. J. Haematol. 92:855-865. - PubMed
-
- Bartram, C. R., W. D. Ludwig, W. Hiddemann, J. Lyons, M. Buschle, J. Ritter, J. Harbott, A. Frohlich, and J. W. Janssen. 1989. Acute myeloid leukemia: analysis of ras gene mutations and clonality defined by polymorphic X-linked loci. Leukemia 3:247-256. - PubMed
-
- Beghini, A., P. Peterlongo, C. B. Ripamonti, L. Larizza, R. Cairoli, E. Morra, and C. Mecucci. 2000. c-kit mutations in core binding factor leukemias. Blood 95:726-727. - PubMed
-
- Bonnet, D., and J. E. Dick. 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3:730-737. - PubMed
-
- Bravo, J., Z. Li, N. A. Speck, and A. J. Warren. 2001. The leukemia-associated AML1 (Runx1)-CBF beta complex functions as a DNA-induced molecular clamp. Nat. Struct. Biol. 8:371-378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
