Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration?
- PMID: 12102510
- PMCID: PMC4233806
- DOI: 10.1093/aob/mcf096
Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration?
Abstract
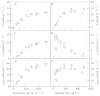
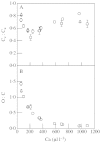
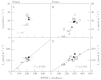
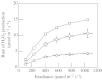
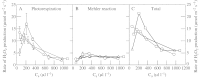
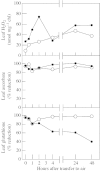
Although active oxygen species are produced at high rates in both the chloroplasts and peroxisomes of the leaves of C3 plants, most attention has focused on the potentially damaging consequences of enhanced chloroplastic production in stress conditions such as drought. This article attempts to provide quantitative estimates of the relative contributions of the chloroplast electron transport chain and the glycolate oxidase reaction to the oxidative load placed on the photosynthetic leaf cell. Rates of photorespiratory H2O2 production were obtained from photosynthetic and photorespiratory flux rates, derived from steady-state leaf gas exchange measurements at varying irradiance and ambient CO2. Assuming a 10% allocation of photosynthetic electron flow to the Mehler reaction, photorespiratory H2O2 production would account for about 70% of total H2O2 formed at all irradiances measured. When chloroplastic CO2 concentration rates are decreased, photorespiration becomes even more predominant in H2O2 generation. At the increased flux through photorespiration observed at lower ambient CO2, the Mehler reaction would have to account for more than 35% of the total photosynthetic electron flow in order to match the rate of peroxisomal H2O2 production. The potential signalling role of H2O2 produced in the peroxisomes is emphasized, and it is demonstrated that photorespiratory H2O2 can perturb the redox states of leaf antioxidant pools. We discuss the interactions between oxidants, antioxidants and redox changes leading to modified gene expression, particularly in relation to drought, and call attention to the potential significance of photorespiratory H2O2 in signalling and acclimation.
Figures
References
-
- AlvarezME, Pennell RI, Meijer P‐J, Ishikawa Am, Dixon RA, Lamb C.1998. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92: 773–784. - PubMed
-
- BartoliCG, Simontacchi M, Tambussi E, Beltrano J, Montaldi E, Puntarulo S.1999. Drought and watering‐dependent oxidative stress: effect on antioxidant content in Triticum aestivum L. leaves. Journal of Experimental Botany 50: 375–383.
-
- CornicG, Briantais J‐M.1991. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 183: 178–184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

