Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought
- PMID: 12102514
- PMCID: PMC4233800
- DOI: 10.1093/aob/mcf064
Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought
Abstract
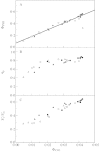
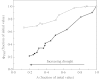
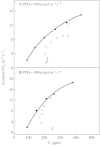
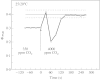
Stomatal closure can explain the inhibition of net CO2 uptake by a leaf subjected to a mild drought: the photosynthetic apparatus appears resistant to lack of water. Changes in both the water content of leaves maintained in a constant environment and the ambient CO2 molar fraction during measurements on well-hydrated leaves lead to similar effects on net CO2 uptake and whole chain electron transport as estimated by leaf chlorophyll fluorescence measurements. In particular, it is shown that photosystem II (PSII) functioning and its regulation are not qualitatively changed during desiccation and that the variations in PSII photochemistry can simply be understood by changes in substrate availability in this condition. Moreover, an analysis of the literature shows that when inhibition of net CO2 uptake by C3 leaves under drought (Phaseolus vulgaris L., Helianthus annus L. and Solanum tuberosum L.) was lower than 80 %, elevated CO2 completely restored the photosynthetic capacity. The CO2 molar fraction in the chloroplasts declines as stomata close in drying leaves. As a consequence, in C3 plants, ribulose-1,5-bisphosphate oxygenation increases and becomes the main sink for photosynthetic electrons. Depending on the prevailing photon flux density, the O2 uptake through photorespiratory activity can entirely replace carbon dioxide as an electron acceptor, or not. The rate of the Mehler reaction remains low and unchanged during desiccation. However, drought could also involve CO2-sensitive modification of the photosynthetic metabolism depending on plant growth conditions and possibly also on plant species.
Figures





References
-
- AsadaK.1999. The water to water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 50: 601–639. - PubMed
-
- BadgerMR.1985. Photosynthetic oxygen‐exchange. Annual Review of Plant Physiology 36: 27–53.
-
- BernacchiCL, Singsaas EL, Pimentel C, Portis Jr AR, Long SP.2001. Improved temperature response functions for models of Rubisco‐limited photosynthesis. Plant Cell and Environment 24: 253–259.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

