Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction
- PMID: 12107102
- PMCID: PMC1850682
- DOI: 10.1016/S0002-9440(10)64169-7
Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction
Abstract
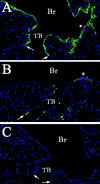
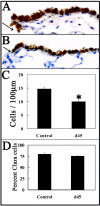
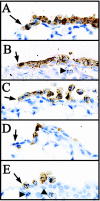
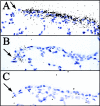
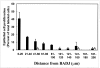
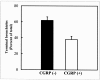
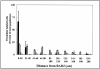
Cellular mechanisms contributing to renewal of terminal bronchioles remain poorly defined. Our previous studies identified pollutant-resistant Clara cell secretory protein (CCSP)-expressing stem cells that localize to the neuroepithelial body (NEB) and contribute to renewal of the proximal bronchiolar epithelium. However, activation of NEB-associated stem cells is unlikely to contribute to renewal of terminal bronchiolar epithelium because of the paucity of NEBs at this location. Goals of this study were to determine the location and properties of cells contributing to renewal of terminal bronchioles after Clara cell depletion. Pollutant-resistant CCSP-expressing cells were identified that localized to the bronchoalveolar duct junction (BADJ) and contribute to restoration of a phenotypically diverse epithelium. CCSP-expressing cells comprise the predominant proliferative population in initial terminal bronchiolar repair and include a population of label-retaining cells suggesting that they maintain characteristics of a stem cell population. Furthermore, immunohistochemical co-localization studies involving CCSP and the NEB-specific marker calcitonin gene-related peptide indicate that BADJ-associated CCSP-expressing stem cells function independently of NEB microenvironments. These studies identify a BADJ-associated, NEB-independent, CCSP-expressing stem cell population in terminal bronchioles and support the notion that regiospecific stem cell niches function to maintain epithelial diversity after injury.
Figures


References
-
- Barth PJ, Wolf M, Ramaswamy A: Distribution and number of Clara cells in the normal and disturbed development of the human fetal lung. Pediatr Pathol 1994, 4:637-651 - PubMed
-
- Plopper CG, Mariassy AT, Hill LH: Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung: I. A comparison of rabbit, guinea pig, rat, hamster, and mouse. Exp Lung Res 1980, 1:139-154 - PubMed
-
- Plopper CG, Mariassy AT, Hill LH: Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung: II. A comparison of horse, steer, sheep, dog, and cat. Exp Lung Res 1980, 1:155-169 - PubMed
-
- Plopper CG, Hill LH, Mariassy AT: Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp Lung Res 1980, 1:171-180 - PubMed
-
- Plopper CG, Mariassy AT, Wilson DW, Alley JL, Nishio SJ, Nettesheim P: Comparison of nonciliated tracheal epithelial cells in six mammalian species: ultrastructure and population densities. Exp Lung Res 1983, 5:281-294 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

