Mycophenolate mofetil inhibits regenerative repair in uranyl acetate-induced acute renal failure by reduced interstitial cellular response
- PMID: 12107106
- PMCID: PMC1850680
- DOI: 10.1016/S0002-9440(10)64173-9
Mycophenolate mofetil inhibits regenerative repair in uranyl acetate-induced acute renal failure by reduced interstitial cellular response
Abstract
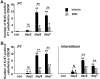
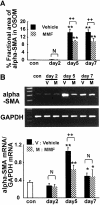
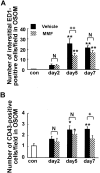
We recently reported that transient appearance of interstitial myofibroblasts and infiltrating macrophages might play a role in cellular recovery in uranyl acetate (UA)-induced acute renal failure (ARF). Here we tested the effects of mycophenolate mofetil (MMF), which attenuates infiltration of lymphocytes, macrophages, and myofibroblasts, but does not suppress epithelial regeneration, on renal tissue repair. Rats treated with MMF (20 mg/kg/day) or vehicle were sacrificed at 2, 5, and 7 days after induction of ARF by injection of 5 mg/kg UA. Renal tissues were immunostained for bromodeoxyuridine (BrdU) and Ki67, alpha-smooth muscle actin (alpha-SMA), ED1, and CD43. The expression levels of alpha-SMA mRNA were examined by reverse transcription-polymerase chain reaction. Body weight loss or serum albumin levels were similar in MMF and vehicle rats during the experiment. In vehicle group, serum creatinine (Scr) significantly increased after day 5, but proximal tubular (PT) damage score increased as early as day 2 after UA injection. BrdU- or Ki67-positive regenerating tubular cells, ED1-positive macrophages and alpha-SMA-positive myofibroblasts significantly increased in the interstitium after day 5. In MMF-treated rats, Scr and PT damage score significantly increased at day 7 and the number of regenerating PT were significantly reduced compared with vehicle-treated rats at days 5 and 7. The numbers of macrophages and myofibroblasts and the expression of alpha-SMA mRNA were significantly lower in MMF than in vehicle rats at day 5, indicating that reduced interstitial cellular response is linked to the inhibition of regenerative repair. CD43-positive lymphocytes were significantly reduced in MMF group than in vehicle group at day 7, suggesting that lymphocyte infiltration does not seem to contribute to early regenerative response of proximal tubules. The transient appearance of myofibroblasts and macrophages in the interstitium may promote regenerative repair in UA-induced ARF in rats.
Figures







Similar articles
-
Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats.Virchows Arch. 2005 Feb;446(2):164-76. doi: 10.1007/s00428-004-1155-5. Epub 2004 Dec 18. Virchows Arch. 2005. PMID: 15609048
-
Possible involvement of myofibroblasts in cellular recovery of uranyl acetate-induced acute renal failure in rats.Am J Pathol. 2000 Oct;157(4):1321-35. doi: 10.1016/S0002-9440(10)64647-0. Am J Pathol. 2000. PMID: 11021836 Free PMC article.
-
Mycophenolate mofetil prevents the progressive renal failure induced by 5/6 renal ablation in rats.Kidney Int. 1999 Mar;55(3):945-55. doi: 10.1046/j.1523-1755.1999.055003945.x. Kidney Int. 1999. PMID: 10027931
-
Regeneration processes in the kidney after acute injury: role of infiltrating cells.Exp Nephrol. 1998 Nov-Dec;6(6):502-7. doi: 10.1159/000020564. Exp Nephrol. 1998. PMID: 9807021 Review.
-
The Role of Tubule-Interstitial Crosstalk in Renal Injury and Recovery.Semin Nephrol. 2020 Mar;40(2):216-231. doi: 10.1016/j.semnephrol.2020.01.012. Semin Nephrol. 2020. PMID: 32303284 Review.
Cited by
-
Temporary changes in macrophages and MHC class-II molecule-expressing cells in the tubulointerstitium in response to uranyl acetate-induced acute renal failure in rats.Virchows Arch. 2003 Aug;443(2):206-16. doi: 10.1007/s00428-003-0839-6. Epub 2003 Jun 13. Virchows Arch. 2003. PMID: 12811555
-
Early activation of fibroblasts is required for kidney repair and regeneration after injury.FASEB J. 2019 Nov;33(11):12576-12587. doi: 10.1096/fj.201900651RR. Epub 2019 Aug 28. FASEB J. 2019. PMID: 31461626 Free PMC article.
-
Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats.Virchows Arch. 2005 Feb;446(2):164-76. doi: 10.1007/s00428-004-1155-5. Epub 2004 Dec 18. Virchows Arch. 2005. PMID: 15609048
-
The therapeutic approaches of renal recovery after relief of the unilateral ureteral obstruction: A comprehensive review.Iran J Basic Med Sci. 2020 Nov;23(11):1367-1373. doi: 10.22038/ijbms.2020.41984.9926. Iran J Basic Med Sci. 2020. PMID: 33235692 Free PMC article. Review.
-
Important role for fibronectin-EIIIA during renal tubular repair and cellular recovery in uranyl acetate-induced acute renal failure of rats.Virchows Arch. 2003 Aug;443(2):194-205. doi: 10.1007/s00428-003-0846-7. Epub 2003 Jul 17. Virchows Arch. 2003. PMID: 12884040
References
-
- Weber KT: Fibrosis, a common pathway to organ failure: angiotensin II and tissue repair. Semin Nephrol 1997, 17:467-491 - PubMed
-
- Badid C, Mounier N, Costa AM, Desmouliere A: Role of myofibroblasts during normal tissue repair and excessive scarring: interest of their assessment in nephropathies. Histol Histopathol 2000, 15:269-280 - PubMed
-
- Muchaneta-Kubara EC, el Nahas AM: Myofibroblast phenotypes expression in experimental renal scarring. Nephrol Dial Transplant 1997, 12:904-915 - PubMed
-
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB: Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 1999, 277:C1-C19 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

