Complicated mechanisms of class II transactivator transcription deficiency in small cell lung cancer and neuroblastoma
- PMID: 12107114
- PMCID: PMC1850684
- DOI: 10.1016/S0002-9440(10)64181-8
Complicated mechanisms of class II transactivator transcription deficiency in small cell lung cancer and neuroblastoma
Abstract
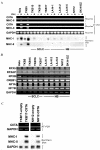
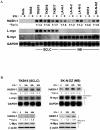
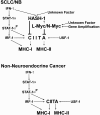
Small cell lung cancer (SCLC) and neuroblastoma (NB), the most aggressive adult and infant neuroendocrine cancers, respectively, are immunologically characterized by a severe reduction in major histocompatibility complex (MHC) that is indispensable for anti-tumor immunity. We had reported that the severe reduction of MHC in SCLC was caused by a deficient interferon (IFN)-gamma-inducible expression of class II transactivator (CIITA) that is known as a very important transcription factor for IFN-gamma-inducible class II and class I MHC expression (Yazawa T, Kamma H, Fujiwara M, Matsui M, Horiguchi H, Satoh H, Fujimoto M, Yokohama K, Ogata T: Lack of class II transactivator causes severe deficiency of HLA-DR expression in small cell lung cancer. J Pathol 1999, 187:191-199). Here, we demonstrate that the reduction of MHC in NB was also caused by a deficient IFN-gamma-inducible expression of CIITA and that the deficiency in SCLC and NB was caused by similar mechanisms. Human achaete-scute complex homologue (HASH)-1, L-myc, and N-myc, which are specifically overexpressed in SCLC and NB, bound to the E-box in CIITA promoter IV and reduced the transcriptional activity. Anti-sense oligonucleotide experiments revealed that overexpressed L-myc and N-myc lie upstream in the regulatory pathway of HASH-1 expression. The expression of HASH-1 was also up-regulated by IFN-gamma. Our results suggest that SCLC and NB have complicated mechanisms of IFN-gamma-inducible CIITA transcription deficiency through the overexpressed HASH-1, L-myc, and N-myc. These complicated mechanisms may play an important role in the escape from anti-tumor immunity.
Figures


Comment in
-
Class II transactivator (CIITA) deficiency in tumor cells: complicated mechanisms or not?Am J Pathol. 2003 Jul;163(1):373-5; author reply 375-6. doi: 10.1016/s0002-9440(10)63664-4. Am J Pathol. 2003. PMID: 12819045 Free PMC article. No abstract available.
Similar articles
-
Lack of class II transactivator causes severe deficiency of HLA-DR expression in small cell lung cancer.J Pathol. 1999 Jan;187(2):191-9. doi: 10.1002/(SICI)1096-9896(199901)187:2<191::AID-PATH206>3.0.CO;2-3. J Pathol. 1999. PMID: 10365094
-
Different levels of control prevent interferon-gamma-inducible HLA-class II expression in human neuroblastoma cells.Oncogene. 2003 Oct 30;22(49):7848-57. doi: 10.1038/sj.onc.1207054. Oncogene. 2003. PMID: 14586411
-
IL-1 beta inhibits IFN-gamma-induced class II MHC expression by suppressing transcription of the class II transactivator gene.J Immunol. 1999 Jan 15;162(2):886-96. J Immunol. 1999. PMID: 9916712
-
Targets in small cell lung cancer.Biochem Pharmacol. 2014 Jan 15;87(2):211-9. doi: 10.1016/j.bcp.2013.09.014. Epub 2013 Sep 30. Biochem Pharmacol. 2014. PMID: 24091017 Review.
-
Amplification and rearrangement of L-myc in human small-cell lung cancer.Mutat Res. 1992 May;276(3):307-15. doi: 10.1016/0165-1110(92)90017-4. Mutat Res. 1992. PMID: 1374523 Review.
Cited by
-
Growth regulation via insulin-like growth factor binding protein-4 and -2 in association with mutant K-ras in lung epithelia.Am J Pathol. 2006 Nov;169(5):1550-66. doi: 10.2353/ajpath.2006.051068. Am J Pathol. 2006. PMID: 17071580 Free PMC article.
-
Insight into Cancer Immunity: MHCs, Immune Cells and Commensal Microbiota.Cells. 2023 Jul 18;12(14):1882. doi: 10.3390/cells12141882. Cells. 2023. PMID: 37508545 Free PMC article. Review.
-
A narrative review of current and potential prognostic biomarkers for immunotherapy in small-cell lung cancer.Ann Transl Med. 2021 May;9(9):809. doi: 10.21037/atm-21-68. Ann Transl Med. 2021. PMID: 34268422 Free PMC article. Review.
-
Regulation of the antigen presentation machinery in cancer and its implication for immune surveillance.Biochem Soc Trans. 2022 Apr 29;50(2):825-837. doi: 10.1042/BST20210961. Biochem Soc Trans. 2022. PMID: 35343573 Free PMC article. Review.
-
Clinical efficacy and safety of immune checkpoint inhibitors plus anlotinib as secondline or subsequent therapy in extensive stage small cell lung cancer: a retrospective study.Clin Transl Oncol. 2025 Mar;27(3):1026-1038. doi: 10.1007/s12094-024-03654-7. Epub 2024 Aug 8. Clin Transl Oncol. 2025. PMID: 39115676
References
-
- Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E: World Health Organization: Histological Typing of Lung and Pleural Tumours, ed 3 1999:pp 1-156 Springer-Verlag, Heidelberg
-
- Schwab M, Shimada H, Joshi V, Brodeur GM: Neuroblastic tumours of adrenal gland and sympathetic nervous system. Kleihues P Cabenee WK eds. Pathology and Genetics of Tumours of the Nervous System. 2000:pp 153-161 International Agency for Research on Cancer, Lyon
-
- Yazawa T, Kamma H, Fujiwara M, Matsui M, Horiguchi H, Satoh H, Fujimoto M, Yokohama K, Ogata T: Lack of class II transactivator causes severe deficiency of HLA-DR expression in small cell lung cancer. J Pathol 1999, 187:191-199 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

