The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis
- PMID: 12107118
- PMCID: PMC1850700
- DOI: 10.1016/s0002-9440(10)64185-5
The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis
Abstract
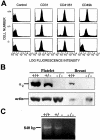
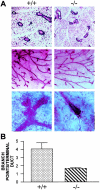
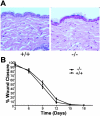
The alpha(2)beta(1) integrin is a collagen/laminin receptor expressed on platelets, endothelial cells, fibroblasts, and epithelial cells. To define the role of the alpha(2)beta(1) integrin in vivo, we created a genetically engineered mouse in which expression of the alpha(2)beta(1) integrin was completely eliminated. Mice deficient in the alpha(2)beta(1) integrin are viable, fertile, and develop normally with no excess lethality of homozygotes. Both alpha(2)beta(1)-integrin protein and alpha(2) mRNA were undetectable in the alpha(2)-null mice. Gross and histological evaluation of the heart, lungs, kidneys, gastrointestinal tract, pancreas, skin, and reproductive tracts revealed no abnormalities. However, quantitative analysis of mammary gland branching morphogenesis demonstrated that branching complexity is markedly diminished in the alpha(2)-deficient animals. Studies in the alpha(2)-deficient animals do not support the proposed roles for the alpha(2)beta(1) integrin on fibroblasts and keratinocytes in wound healing. When compared to platelets from wild-type littermates, platelets from alpha(2)-null mice failed to adhere to type I collagen under either static or shear-stress conditions. Although platelets from alpha(2)-deficient animals aggregated in response to collagen, they did so with prolonged lag time and lessened intensity. The alpha(2)beta(1) integrin-null mouse thus exhibits diverse, sometimes subtle, phenotypes consistent with the widespread pattern of alpha(2)beta(1) integrin expression.
Figures


Comment in
-
Lessons from the alpha2 integrin knockout mouse.Am J Pathol. 2002 Jul;161(1):3-6. doi: 10.1016/s0002-9440(10)64149-1. Am J Pathol. 2002. PMID: 12107082 Free PMC article. Review. No abstract available.
References
-
- Kirchhofer D, Languino LR, Ruoslahti E, Pierschbacher MD: Alpha 2 beta 1 integrins from different cell types show different binding specificities. J Biol Chem 1990, 265:615-618 - PubMed
-
- Santoro SA, Zutter MM: The alpha 2 beta 1 integrin: a collagen receptor on platelets and other cells. Thromb Haemost 1995, 74:813-821 - PubMed
-
- Berdichevsky F, Alford D, D’Souza B, Taylor-Papadimitriou J: Branching morphogenesis of human mammary epithelial cells in collagen gels. J Cell Sci 1994, 107:3557-3568 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

