Engineering of tissue inhibitor of metalloproteinases mutants as potential therapeutics
- PMID: 12110123
- PMCID: PMC3240149
- DOI: 10.1186/ar573
Engineering of tissue inhibitor of metalloproteinases mutants as potential therapeutics
Abstract
Matrix metalloproteinases (MMPs) play a central role in many biological processes such as development, morphogenesis and wound healing, but their unbalanced activities are implicated in numerous disease processes such as arthritis, cancer metastasis, atherosclerosis, nephritis and fibrosis. One of the key mechanisms to control MMP activities is inhibition by endogenous inhibitors called tissue inhibitors of metalloproteinases (TIMPs). This review highlights the structures and inhibition mechanism of TIMPs, the biological activities of TIMPs, the unique properties of TIMP-3, and the altered specificity towards MMPs achieved by mutagenesis. A potential therapeutic use of TIMP variants is discussed.
Figures
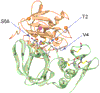



Similar articles
-
Tissue inhibitors of metalloproteinases: evolution, structure and function.Biochim Biophys Acta. 2000 Mar 7;1477(1-2):267-83. doi: 10.1016/s0167-4838(99)00279-4. Biochim Biophys Acta. 2000. PMID: 10708863 Review.
-
Designing TIMP (tissue inhibitor of metalloproteinases) variants that are selective metalloproteinase inhibitors.Biochem Soc Symp. 2003;(70):201-12. doi: 10.1042/bss0700201. Biochem Soc Symp. 2003. PMID: 14587293 Review.
-
Structure and function of matrix metalloproteinases and TIMPs.Cardiovasc Res. 2006 Feb 15;69(3):562-73. doi: 10.1016/j.cardiores.2005.12.002. Epub 2006 Jan 5. Cardiovasc Res. 2006. PMID: 16405877 Review.
-
The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity.Biochim Biophys Acta. 2010 Jan;1803(1):55-71. doi: 10.1016/j.bbamcr.2010.01.003. Epub 2010 Jan 15. Biochim Biophys Acta. 2010. PMID: 20080133 Free PMC article. Review.
-
Dynamic interdomain interactions contribute to the inhibition of matrix metalloproteinases by tissue inhibitors of metalloproteinases.J Biol Chem. 2011 Jun 10;286(23):21002-12. doi: 10.1074/jbc.M110.200139. Epub 2011 Apr 25. J Biol Chem. 2011. PMID: 21518756 Free PMC article.
Cited by
-
Matrix metalloproteinases as breast cancer drivers and therapeutic targets.Front Biosci (Landmark Ed). 2015 Jun 1;20(7):1144-63. doi: 10.2741/4364. Front Biosci (Landmark Ed). 2015. PMID: 25961550 Free PMC article. Review.
-
Resveratrol Inhibits MMP3 and MMP9 Expression and Secretion by Suppressing TLR4/NF-κB/STAT3 Activation in Ox-LDL-Treated HUVECs.Oxid Med Cell Longev. 2019 Sep 10;2019:9013169. doi: 10.1155/2019/9013169. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31583048 Free PMC article.
-
MMP-3 activation is involved in copper oxide nanoparticle-induced epithelial-mesenchymal transition in human lung epithelial cells.Nanotoxicology. 2021 Dec;15(10):1380-1402. doi: 10.1080/17435390.2022.2030822. Epub 2022 Feb 2. Nanotoxicology. 2021. PMID: 35108494 Free PMC article.
-
Human neutrophil elastase-mediated cleavage sites of MMP-9 and TIMP-1: implications to cystic fibrosis proteolytic dysfunction.Mol Med. 2010 May-Jun;16(5-6):159-66. doi: 10.2119/molmed.2009.00109. Epub 2010 Jan 22. Mol Med. 2010. PMID: 20111696 Free PMC article.
-
Influence of single nucleotide polymorphisms (SNPs) in genetic susceptibility towards periprosthetic osteolysis.Genes Genomics. 2019 Oct;41(10):1113-1125. doi: 10.1007/s13258-019-00845-3. Epub 2019 Jul 16. Genes Genomics. 2019. PMID: 31313107 Review.
References
-
- Poole AR, Mort JS, Roughley PJ. In: In Joint Cartilage Degradation: Basic and Clinical Aspects. Edited by Woessner JF Jr, Howell DS., editor. New York: Marcel Dekker;; 1993. Methods for evaluating mechanisms of cartilage breakdown. pp. 225–260.
-
- Konttinen YT, Ainola M, Valleala H, Ma J, Ida H, Mandelin J, Kinne RW, Santavirta S, Sorsa T, López-Otín C, Takagi M. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis. 1999;58:691–697. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

