Alternative splicing modulates the frequency-dependent response of CaMKII to Ca(2+) oscillations
- PMID: 12110572
- PMCID: PMC126106
- DOI: 10.1093/emboj/cdf360
Alternative splicing modulates the frequency-dependent response of CaMKII to Ca(2+) oscillations
Abstract
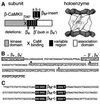
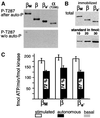
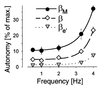
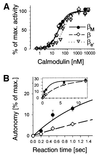
Ca(2+) oscillations are required in various signal trans duction pathways, and contain information both in their amplitude and frequency. Remarkably, the Ca(2+)/calmodulin(CaM)-dependent protein kinase II (CaMKII) can decode such frequencies. A Ca(2+)/CaM-stimulated autophosphorylation leads to Ca(2+)/CaM-independent (autonomous) activity of the kinase that outlasts the initial stimulation. This autonomous activity increases exponentially with the frequency of Ca(2+) oscillations. Here we show that three beta-CaMKII splice variants (beta(M), beta and beta(e)') have very similar specific activity and maximal autonomy. However, their autonomy generated by Ca(2+) oscillations differs significantly. A mechanistic basis was found in alterations of the CaM activation constant and of the initial rate of autophosphorylation. Structurally, the splice variants differ only in a variable 'linker' region between the kinase and association domains. Therefore, we propose that differences in relative positioning of kinase domains within multimeric holoenzymes are responsible for the observed effects. Notably, the beta-CaMKII splice variants are differentially expressed, even among individual hippocampal neurons. Taken together, our results suggest that alternative splicing provides cells with a mechanism to modulate their sensitivity to Ca(2+) oscillations.
Figures
References
-
- Bayer K.U. and Schulman,H. (2001) Regulation of signal transduction by protein targeting: the case for CaMKII. Biochem. Biophys. Res. Commun., 289, 917–923. - PubMed
-
- Bayer K.U., Löhler,J., Schulman,H. and Harbers,K. (1999) Develop mental expression of the CaM kinase II isoforms: ubiquitous γ- and δ-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Mol. Brain Res., 70, 147–154. - PubMed
-
- Bayer K.U., De Koninck,P., Leonard,A.S., Hell,J.W. and Schulman,H. (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature, 411, 801–805. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

