c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis
- PMID: 12110583
- PMCID: PMC125398
- DOI: 10.1093/emboj/cdf356
c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis
Abstract
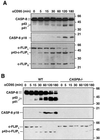
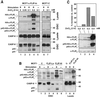
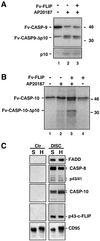
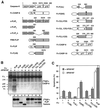
Activation of the caspase cascade is a pivotal step in apoptosis and can occur via death adaptor-mediated homo-oligomerization of initiator procaspases. Here we show that c-FLIP(L), a protease-deficient caspase homolog widely regarded as an apoptosis inhibitor, is enriched in the CD95 death-inducing signaling complex (DISC) and potently promotes procaspase-8 activation through hetero-dimerization. c-FLIP(L) exerts its effect through its protease-like domain, which associates efficiently with the procaspase-8 protease domain and induces the enzymatic activity of the zymogen. Ectopic expression of c-FLIP(L) at physiologically relevant levels enhances procaspase-8 processing in the CD95 DISC and promotes apoptosis, while a decrease of c-FLIP(L) expression results in inhibition of apoptosis. c-FLIP(L) acts as an apoptosis inhibitor only at high ectopic expression levels. Thus, c-FLIP(L) defines a novel type of caspase regulator, distinct from the death adaptors, that can either promote or inhibit apoptosis.
Figures



References
-
- Goltsev Y.V., Kovalenko,A.V., Arnold,E., Varfolomeev,E.E., Brodianskii,V.M. and Wallach,D. (1997) CASH, a novel caspase homologue with death effector domains. J. Biol. Chem., 272, 19641–19644. - PubMed
-
- Griffith T.S., Chin,W.A., Jackson,G.C., Lynch,D.H. and Kubin,M.Z. (1998) Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J. Immunol., 161, 2833–2840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

