FcepsilonRI cross-linking-induced actin assembly mediates calcium signalling in RBL-2H3 mast cells
- PMID: 12110608
- PMCID: PMC1573417
- DOI: 10.1038/sj.bjp.0704788
FcepsilonRI cross-linking-induced actin assembly mediates calcium signalling in RBL-2H3 mast cells
Abstract
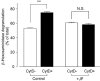
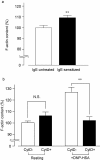
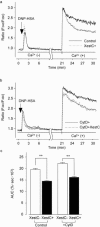
1. To determine the role of actin assembly in the Ca(2+) signalling of mast cells activated by cross-linking of FcepsilonRI, we examined the effects of cytochalasin D, an inhibitor of actin polymerization. 2. In the RBL-2H3 cells, F-actin content was increased by sensitization with anti-dinitrophenol (DNP) IgE. In these cells, cytochalasin D induced oscillatory increases in cytosolic Ca(2+) ([Ca(2+)](i)); these increase were inhibited by jasplakinolide, a stabilizer of actin filaments. 3. In the IgE-sensitized RBL-2H3 cells, DNP-human serum albumin (DNP-HSA) augmented actin assembly. DNP-HSA also increased the production of IP(3), [Ca(2+)](i) and degranulation. Cytochalasin D enhanced all of these DNP-HSA-induced effects. 4. In a Ca(2+)-free solution, DNP-HSA induced a transient increase in [Ca(2+)](i), and this increase was accelerated by cytochalasin D. After cessation of the DNP-HSA-induced Ca(2+) release, the re-addition of Ca(2+) induced a sustained increase in [Ca(2+)](i) through capacitative Ca(2+) entry (CCE), and this increase was enhanced by cytochalasin D. 5 The effect of cytochalasin D in enhancing the CCE activity was prevented by xestospongin C. 6. In contrast, neither the Ca(2+) release nor the CCE activation that was induced by thapsigargin was affected by cytochalasin D. 7. These results suggest that actin de-polymerization stimulates the FcepsilonRI-mediated signalling to augment the release of Ca(2+) from the endoplasmic reticulum in RBL-2H3 cells.
British Journal of Pharmacology (2002) 136, 837-846
Figures



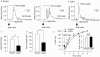

Similar articles
-
Xestospongin C, a novel blocker of IP3 receptor, attenuates the increase in cytosolic calcium level and degranulation that is induced by antigen in RBL-2H3 mast cells.Br J Pharmacol. 2002 Apr;135(8):1959-66. doi: 10.1038/sj.bjp.0704662. Br J Pharmacol. 2002. PMID: 11959799 Free PMC article.
-
The role of actin microfilaments in the down-regulation of the degranulation response in RBL-2H3 mast cells.J Immunol. 1999 Feb 15;162(4):2243-50. J Immunol. 1999. PMID: 9973500
-
Fluvastatin inhibits mast cell degranulation without changing the cytoplasmic Ca2+ level.Eur J Pharmacol. 2009 Jan 14;602(2-3):432-8. doi: 10.1016/j.ejphar.2008.11.040. Epub 2008 Nov 27. Eur J Pharmacol. 2009. PMID: 19059394
-
Calcium signaling in mast cells: focusing on L-type calcium channels.Adv Exp Med Biol. 2012;740:955-77. doi: 10.1007/978-94-007-2888-2_44. Adv Exp Med Biol. 2012. PMID: 22453979 Review.
-
Jasplakinolide. An actin-specific reagent that promotes actin polymerization.Methods Mol Biol. 2001;161:109-20. doi: 10.1385/1-59259-051-9:109. Methods Mol Biol. 2001. PMID: 11190499 Review. No abstract available.
Cited by
-
Focal adhesion proteins connect IgE receptors to the cytoskeleton as revealed by micropatterned ligand arrays.Proc Natl Acad Sci U S A. 2008 Nov 11;105(45):17238-44. doi: 10.1073/pnas.0802138105. Proc Natl Acad Sci U S A. 2008. PMID: 19004813 Free PMC article.
-
Suppression of FcεRI-evoked Degranulation in RBL-2H3 Cells on Gelatin Methacryloyl Hydrogel.Cell Biochem Biophys. 2025 Jun;83(2):2481-2488. doi: 10.1007/s12013-024-01657-3. Epub 2024 Dec 28. Cell Biochem Biophys. 2025. PMID: 39731647
-
Inhibitory mechanism of xestospongin-C on contraction and ion channels in the intestinal smooth muscle.Br J Pharmacol. 2002 Dec;137(8):1207-12. doi: 10.1038/sj.bjp.0704988. Br J Pharmacol. 2002. PMID: 12466229 Free PMC article.
-
p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics.Blood. 2009 Mar 19;113(12):2695-705. doi: 10.1182/blood-2008-06-160861. Epub 2009 Jan 5. Blood. 2009. PMID: 19124833 Free PMC article.
-
Mast cell desensitization inhibits calcium flux and aberrantly remodels actin.J Clin Invest. 2016 Nov 1;126(11):4103-4118. doi: 10.1172/JCI87492. Epub 2016 Sep 26. J Clin Invest. 2016. PMID: 27669462 Free PMC article.
References
-
- AKETANI S., TESHIMA R., UMEZAWA Y., SAWADA J. Correlation between cytosolic calcium concentration and degranulation in RBL-2H3 cells in the presence of various concentrations of antigen-specific IgEs. Immunol. Lett. 2001;75:185–189. - PubMed
-
- BARKALOW K., HARTWIG J.H. The role of actin filament barbed-end exposure in cytoskeletal dynamics and cell motility. Biochem. Soc. Trans. 1995;23:451–456. - PubMed
-
- BERRIDGE M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- CARLIER M.F., PANTALONI D. Control of actin dynamics in cell motility. J. Mol. Biol. 1997;269:459–467. - PubMed
-
- CLAPHAM D.E. Intracellular calcium. Replenishing the stores. Nature. 1995;375:634–635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

