Different actions of protein kinase C isoforms alpha and epsilon on gastric acid secretion
- PMID: 12110618
- PMCID: PMC1573419
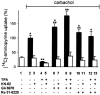
- DOI: 10.1038/sj.bjp.0704790
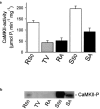
Different actions of protein kinase C isoforms alpha and epsilon on gastric acid secretion
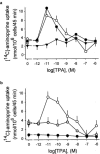
Abstract
1. The phorbol ester TPA, an activator of protein kinase C (PKC), inhibits cholinergic stimulation of gastric acid secretion but increases basal H(+) secretion. 2. Since these contradictory findings suggest the action of different PKC isozymes we analysed the role of calcium-dependent PKC-alpha, and calcium-independent PKC-epsilon in gastric acid secretion. 3. Inhibition of PKC-alpha by the indolocarbazole Gö 6976 revealed that about 28% of carbachol-induced acid secretion was inhibited by PKC-alpha. In the presence of Gö 6976 approximately 64% of the carbachol-induced signal transduction is mediated by Ca(2+)/calmodulin-dependent protein kinase II (CaMKII), and 14% is conveyed by PKC-epsilon as deduced from the inhibition with the bisindolylmaleimide Ro 31-8220. 4. Inhibition of carbachol-induced acid secretion by TPA was accompanied by a decrease in CaMKII activity. 5. The stimulation of basal acid secretion by TPA was biphasic with a peak at a very low concentration (10 pM), resulting in an activation of the calcium-sensor CaMKII. The activation was determined with a phosphospecific polyclonal antibody against active CaMKII. The TPA-induced increase of H(+) secretion was sensitive to the cell-permeable Ca(2+)-chelator BAPTA/AM, Ro 31-8220, and the CaMKII-inhibitor KN-62, but not to Gö 6976. 6. Since TPA induced the translocation of PKC-epsilon but not of PKC-alpha in resting parietal cells, PKC-epsilon seems to be at least responsible for an initial elevation of free intracellular calcium to initiate TPA-induced acid secretion. 7. Our data indicate the different roles of two PKC isoforms: PKC-epsilon activation appears to facilitate cholinergic stimulation of H(+)-secretion likely by increasing intracellular calcium. In contrast, PKC-alpha activation attenuates acid secretion accompanied by a down-regulation of CaMKII activity.
British Journal of Pharmacology (2002) 136, 938-946
Figures




References
-
- AKITA Y., OHNO S., YAJIMA Y., KONNO Y., SAIDO T.C., MIZUNO K., CHIDA K., OSADA S., KUROKI T., KAWASHIMA S., SUZUKI K. Overproduction of a Ca(2+)-independent protein kinase C isozyme, nPKC epsilon, increases the secretion of prolactin from thyrotropin-releasing hormone-stimulated rat pituitary GH4C1 cells. J. Biol. Chem. 1994;269:4653–4660. - PubMed
-
- BARANSKA J., CHABAN V., CZARNY M., SABALA P. Changes in Ca2+ concentration in phorbolester and thapsigargin treated glioma C6 cells. The role of protein kinase C in regulation of Ca2+ entry. Cell Calcium. 1995;17:207–215. - PubMed
-
- BASTANI B., YANG L., BADASSASARE J.J., POLLO D.A., GARDNER J.D. Cellular distribution of isoforms of protein kinase C (PKC) in pancreatic acini. Biochim. Biophys. Acta. 1995;1269:307–315. - PubMed
-
- BEIL W., MANNSCHEDEL W., SEWING K.F. Protein kinase C and parietal cell function. Biochem. Biophys. Res. Commun. 1987;149:720–728. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

